NSCLC Cells Resistance to PI3K/mTOR Inhibitors Is Mediated by Delta-6 Fatty Acid Desaturase (FADS2)
- PMID: 36496978
- PMCID: PMC9736998
- DOI: 10.3390/cells11233719
NSCLC Cells Resistance to PI3K/mTOR Inhibitors Is Mediated by Delta-6 Fatty Acid Desaturase (FADS2)
Abstract
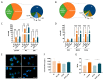
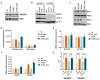
Hyperactivation of the phosphatidylinositol-3-kinase (PI3K) pathway is one of the most common events in human cancers. Several efforts have been made toward the identification of selective PI3K pathway inhibitors. However, the success of these molecules has been partially limited due to unexpected toxicities, the selection of potentially responsive patients, and intrinsic resistance to treatments. Metabolic alterations are intimately linked to drug resistance; altered metabolic pathways can help cancer cells adapt to continuous drug exposure and develop resistant phenotypes. Here we report the metabolic alterations underlying the non-small cell lung cancer (NSCLC) cell lines resistant to the usual PI3K-mTOR inhibitor BEZ235. In this study, we identified that an increased unsaturation degree of lipid species is associated with increased plasma membrane fluidity in cells with the resistant phenotype and that fatty acid desaturase FADS2 mediates the acquisition of chemoresistance. Therefore, new studies focused on reversing drug resistance based on membrane lipid modifications should consider the contribution of desaturase activity.
Keywords: BEZ235; PI3K/Akt/mTOR pathway; drug resistance; lipid metabolism; non-small-cell lung cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

