Single-Cell Sequencing Reveals the Regulatory Role of Maresin1 on Neutrophils during Septic Lung Injury
- PMID: 36496993
- PMCID: PMC9739442
- DOI: 10.3390/cells11233733
Single-Cell Sequencing Reveals the Regulatory Role of Maresin1 on Neutrophils during Septic Lung Injury
Abstract
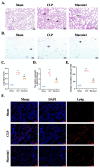
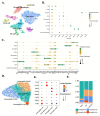
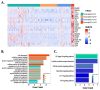
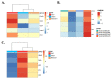
Acute lung injury (ALI) is the most common type of organ injury in sepsis, with high morbidity and mortality. Sepsis is characterized by an inappropriate inflammatory response while neutrophils exert an important role in the excessive inflammatory response. The discovery of specialized pro-resolving mediators (SPMs) provides a new direction for the treatment of a series of inflammatory-related diseases including sepsis. Among them, the regulation of Maresin1 on immune cells was widely demonstrated. However, current research on the regulatory effects of Maresin1 on immune cells has remained at the level of certain cell types. Under inflammatory conditions, the immune environment is complex and immune cells exhibit obvious heterogeneity. Neutrophils play a key role in the occurrence and development of septic lung injury. Whether there is a subpopulation bias in the regulation of neutrophils by Maresin1 has not been elucidated. Therefore, with the well-established cecal ligation and puncture (CLP) model and single-cell sequencing technology, our study reveals for the first time the regulatory mechanism of Maresin1 on neutrophils at the single-cell level. Our study suggested that Maresin1 can significantly reduce neutrophil infiltration in septic lung injury and that this regulatory effect is more concentrated in the Neutrophil-Cxcl3 subpopulation. Maresin1 can significantly reduce the infiltration of the Neutrophil-Cxcl3 subpopulation and inhibit the expression of related inflammatory genes and key transcription factors in the Neutrophil-Cxcl3 subpopulation. Our study provided new possibilities for specific modulation of neutrophil function in septic lung injury.
Keywords: Maresin1; cellular heterogeneity; neutrophils; sepsis-induced lung injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Maresin1 ameliorates acute lung injury induced by sepsis through regulating Th17/Treg balance.Life Sci. 2020 Aug 1;254:117773. doi: 10.1016/j.lfs.2020.117773. Epub 2020 May 11. Life Sci. 2020. PMID: 32418896
-
CD177+ neutrophils exacerbate septic lung injury via the NETs/AIM2 pathway: An experimental and bioinformatics study.Int Immunopharmacol. 2025 Apr 4;151:114292. doi: 10.1016/j.intimp.2025.114292. Epub 2025 Feb 24. Int Immunopharmacol. 2025. PMID: 40007380
-
Maresin1 ameliorates sepsis-associated lung injury by inhibiting the activation of the JAK2/STAT3 and MAPK/ NF-κB signaling pathways.Microb Pathog. 2020 Nov;148:104468. doi: 10.1016/j.micpath.2020.104468. Epub 2020 Aug 29. Microb Pathog. 2020. PMID: 32866582
-
The dual role of neutrophils in sepsis-associated liver injury.Front Immunol. 2025 Feb 28;16:1538282. doi: 10.3389/fimmu.2025.1538282. eCollection 2025. Front Immunol. 2025. PMID: 40092997 Free PMC article. Review.
-
Maresin1 can be a potential therapeutic target for nerve injury.Biomed Pharmacother. 2023 May;161:114466. doi: 10.1016/j.biopha.2023.114466. Epub 2023 Mar 2. Biomed Pharmacother. 2023. PMID: 36870281 Review.
Cited by
-
The potential of exogenous specialized pro-resolving mediators in protecting against sepsis-associated lung injury: a review.Front Pharmacol. 2025 Jul 29;16:1622754. doi: 10.3389/fphar.2025.1622754. eCollection 2025. Front Pharmacol. 2025. PMID: 40799817 Free PMC article. Review.
-
Heterogeneous Patterns of Endothelial NF-κB p65 and MAPK c-Jun Activation, Adhesion Molecule Expression, and Leukocyte Recruitment in Lung Microvasculature of Mice with Sepsis.Biomedicines. 2024 Jul 26;12(8):1672. doi: 10.3390/biomedicines12081672. Biomedicines. 2024. PMID: 39200137 Free PMC article.
-
Advances in nanomaterial-targeted treatment of acute lung injury after burns.J Nanobiotechnology. 2024 Jun 18;22(1):342. doi: 10.1186/s12951-024-02615-0. J Nanobiotechnology. 2024. PMID: 38890721 Free PMC article. Review.
-
Identification of novel biomarkers related to neutrophilic inflammation in COPD.Front Immunol. 2024 May 30;15:1410158. doi: 10.3389/fimmu.2024.1410158. eCollection 2024. Front Immunol. 2024. PMID: 38873611 Free PMC article.
References
-
- Fleischmann C., Scherag A., Adhikari N.K., Hartog C.S., Tsaganos T., Schlattmann P., Angus D.C., Reinhart K. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016;193:259–272. doi: 10.1164/rccm.201504-0781OC. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

