Cell Sources for Retinal Regeneration: Implication for Data Translation in Biomedicine of the Eye
- PMID: 36497013
- PMCID: PMC9738527
- DOI: 10.3390/cells11233755
Cell Sources for Retinal Regeneration: Implication for Data Translation in Biomedicine of the Eye
Abstract
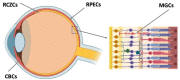
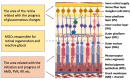
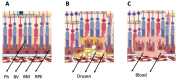
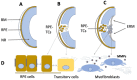
The main degenerative diseases of the retina include macular degeneration, proliferative vitreoretinopathy, retinitis pigmentosa, and glaucoma. Novel approaches for treating retinal diseases are based on cell replacement therapy using a variety of exogenous stem cells. An alternative and complementary approach is the potential use of retinal regeneration cell sources (RRCSs) containing retinal pigment epithelium, ciliary body, Müller glia, and retinal ciliary region. RRCSs in lower vertebrates in vivo and in mammals mostly in vitro are able to proliferate and exhibit gene expression and epigenetic characteristics typical for neural/retinal cell progenitors. Here, we review research on the factors controlling the RRCSs' properties, such as the cell microenvironment, growth factors, cytokines, hormones, etc., that determine the regenerative responses and alterations underlying the RRCS-associated pathologies. We also discuss how the current data on molecular features and regulatory mechanisms of RRCSs could be translated in retinal biomedicine with a special focus on (1) attempts to obtain retinal neurons de novo both in vivo and in vitro to replace damaged retinal cells; and (2) investigations of the key molecular networks stimulating regenerative responses and preventing RRCS-related pathologies.
Keywords: intrinsic cell sources; ophthalmotherapy; regulatory network; retinal degenerative diseases; retinal regeneration.
Conflict of interest statement
The author declares no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The author states that the manuscript has not been published previously.
Figures
Similar articles
-
Potential Endogenous Cell Sources for Retinal Regeneration in Vertebrates and Humans: Progenitor Traits and Specialization.Biomedicines. 2020 Jul 12;8(7):208. doi: 10.3390/biomedicines8070208. Biomedicines. 2020. PMID: 32664635 Free PMC article. Review.
-
Insights on the Regeneration Potential of Müller Glia in the Mammalian Retina.Cells. 2021 Jul 31;10(8):1957. doi: 10.3390/cells10081957. Cells. 2021. PMID: 34440726 Free PMC article. Review.
-
Molecular characterization of retinal stem cells and their niches in adult zebrafish.BMC Dev Biol. 2006 Jul 26;6:36. doi: 10.1186/1471-213X-6-36. BMC Dev Biol. 2006. PMID: 16872490 Free PMC article.
-
Lin28b stimulates the reprogramming of rat Müller glia to retinal progenitors.Exp Cell Res. 2017 Mar 1;352(1):164-174. doi: 10.1016/j.yexcr.2017.02.010. Epub 2017 Feb 9. Exp Cell Res. 2017. PMID: 28189638
-
Retinal Stem Cell 'Retirement Plans': Growth, Regulation and Species Adaptations in the Retinal Ciliary Marginal Zone.Int J Mol Sci. 2021 Jun 18;22(12):6528. doi: 10.3390/ijms22126528. Int J Mol Sci. 2021. PMID: 34207050 Free PMC article. Review.
Cited by
-
Recent Achievements in the Heterogeneity of Mammalian and Human Retinal Pigment Epithelium: In Search of a Stem Cell.Cells. 2024 Feb 4;13(3):281. doi: 10.3390/cells13030281. Cells. 2024. PMID: 38334673 Free PMC article. Review.
-
Epigenetic Modifications in the Retinal Pigment Epithelium of the Eye During RPE-Related Regeneration or Retinal Diseases in Vertebrates.Biomedicines. 2025 Jun 25;13(7):1552. doi: 10.3390/biomedicines13071552. Biomedicines. 2025. PMID: 40722628 Free PMC article. Review.
-
Retinal Ganglion Cell Replacement in Glaucoma Therapy: A Narrative Review.J Clin Med. 2024 Nov 27;13(23):7204. doi: 10.3390/jcm13237204. J Clin Med. 2024. PMID: 39685661 Free PMC article. Review.
-
Impact of Microgravity and Other Spaceflight Factors on Retina of Vertebrates and Humans In Vivo and In Vitro.Life (Basel). 2023 May 26;13(6):1263. doi: 10.3390/life13061263. Life (Basel). 2023. PMID: 37374046 Free PMC article. Review.
References
-
- Varma R., Vajaranant T.S., Burkemper B., Wu S., Torres M., Hsu C., Choudhury F., McKean-Codwin P. Visual impairment and blindness in adults in the United States: Demographic and geographic variations from 2015 to 2050. JAMA Ophthalmol. 2016;134:802–809. doi: 10.1001/jamaophthalmol.2016.1284. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

