Type XXII Collagen Complements Fibrillar Collagens in the Serological Assessment of Tumor Fibrosis and the Outcome in Pancreatic Cancer
- PMID: 36497023
- PMCID: PMC9738409
- DOI: 10.3390/cells11233763
Type XXII Collagen Complements Fibrillar Collagens in the Serological Assessment of Tumor Fibrosis and the Outcome in Pancreatic Cancer
Abstract
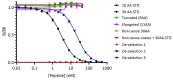
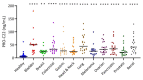
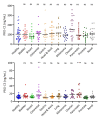
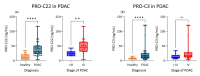
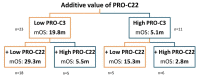
Circulating fragments of type III collagen, measured by PRO-C3, has shown promising results as a tumor fibrosis biomarker. However, the fibrotic tumor microenvironment consists of many other collagens with diverse functions and unexplored biomarker potential. One example hereof is type XXII collagen (COL22). In this study, we investigated the biomarker potential of COL22 by measuring this in serum. An ELISA, named PRO-C22, was developed and measured in two serum cohorts consisting of patients with various solid tumors (n = 220) and healthy subjects (n = 33) (Cohort 1), and patients with pancreatic ductal adenocarcinoma (PDAC) (n = 34), and healthy subjects (n = 20) (Cohort 2). In Cohort 1, PRO-C22 was elevated in the serum from patients with solid tumors, compared to healthy subjects (p < 0.01 to p < 0.0001), and the diagnostic accuracy (AUROC) ranged from 0.87 to 0.98, p < 0.0001. In Cohort 2, the high levels of PRO-C22, in patients with PDAC, were predictive of a worse overall survival (HR = 4.52, 95% CI 1.90−10.7, p = 0.0006) and this remained significant after adjusting for PRO-C3 (HR = 4.27, 95% CI 1.24−10.4, p = 0.0013). In conclusion, PRO-C22 has diagnostic biomarker potential in various solid tumor types and prognostic biomarker potential in PDAC. Furthermore, PRO-C22 complemented PRO-C3 in predicting mortality, suggesting an additive prognostic value when quantifying different collagens.
Keywords: ECM; PDAC; collagens; non-invasive biomarker; tumor fibrosis; type XXII collagen.
Conflict of interest statement
A patent has been filed for the PRO-C22 ELISA. E.A.M., J.T.-U., C.J., N.I.N., M.A.K. and N.W. are employed at Nordic Bioscience A/S, a biotech company involved in the discovery and development of blood-based biomarkers. I.M.C., J.S.J., H.M.H.D., L.N.J. and C.P.H., have no conflict of interest to declare. E.A.M., M.A.K. and N.W. own stock in Nordic Bioscience A/S.
Figures

References
-
- WHO WHO|Cancer Factsheet. [(accessed on 23 July 2021)]; Available online: http://www.who.int/mediacentre/factsheets/fs297/en/
-
- World Health Organization . WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All. World Health Organization; Geneva, Switzerland: 2020.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

