Vascularization of Poly-ε-Caprolactone-Collagen I-Nanofibers with or without Sacrificial Fibers in the Neurotized Arteriovenous Loop Model
- PMID: 36497034
- PMCID: PMC9736129
- DOI: 10.3390/cells11233774
Vascularization of Poly-ε-Caprolactone-Collagen I-Nanofibers with or without Sacrificial Fibers in the Neurotized Arteriovenous Loop Model
Abstract
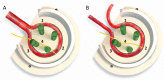
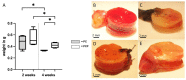
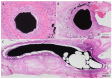
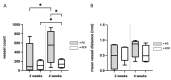
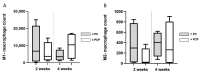
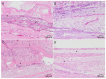
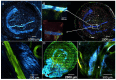
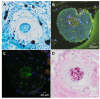
Electrospun nanofibers represent an ideal matrix for the purpose of skeletal muscle tissue engineering due to their highly aligned structure in the nanoscale, mimicking the extracellular matrix of skeletal muscle. However, they often consist of high-density packed fibers, which might impair vascularization. The integration of polyethylene oxide (PEO) sacrificial fibers, which dissolve in water, enables the creation of less dense structures. This study examines potential benefits of poly-ε-caprolactone-collagen I-PEO-nanoscaffolds (PCP) in terms of neovascularization and distribution of newly formed vessels compared to poly-ε-caprolactone -collagen I-nanoscaffolds (PC) in a modified arteriovenous loop model in the rat. For this purpose, the superficial inferior epigastric artery and vein as well as a motor nerve branch were integrated into a multilayer three-dimensional nanofiber scaffold construct, which was enclosed by an isolation chamber. Numbers and spatial distribution of sprouting vessels as well as macrophages were analyzed via immunohistochemistry after two and four weeks of implantation. After four weeks, aligned PC showed a higher number of newly formed vessels, regardless of the compartments formed in PCP by the removal of sacrificial fibers. Both groups showed cell influx and no difference in macrophage invasion. In this study, a model of combined axial vascularization and neurotization of a PCL-collagen I-nanofiber construct could be established for the first time. These results provide a foundation for the in vivo implantation of cells, taking a major step towards the generation of functional skeletal muscle tissue.
Keywords: AV loop model; EPI loop model; PCL-collagen I-nanofiber scaffolds; neoangiogenesis; neurotization; polyethylene oxide; vascularization of nanofiber scaffolds.
Conflict of interest statement
The authors declare no conflict of interest. Any influence of the funding sources on the study design, analysis and interpretation of the results can be excluded by the authors. No benefit of any kind will be received either directly or indirectly by the authors.
Figures


References
-
- Cai A., Hardt M., Schneider P., Schmid R., Lange C., Dippold D., Schubert D.W., Boos A.M., Weigand A., Arkudas A., et al. Myogenic differentiation of primary myoblasts and mesenchymal stromal cells under serum-free conditions on PCL-collagen I-nanoscaffolds. BMC Biotechnol. 2018;18:75. doi: 10.1186/s12896-018-0482-6. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

