SARS-CoV-2-Associated ssRNAs Activate Human Neutrophils in a TLR8-Dependent Fashion
- PMID: 36497044
- PMCID: PMC9738506
- DOI: 10.3390/cells11233785
SARS-CoV-2-Associated ssRNAs Activate Human Neutrophils in a TLR8-Dependent Fashion
Abstract
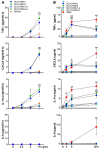
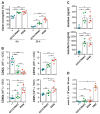
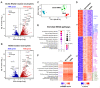
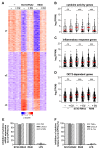
COVID-19 disease is characterized by a dysregulation of the innate arm of the immune system. However, the mechanisms whereby innate immune cells, including neutrophils, become activated in patients are not completely understood. Recently, we showed that GU-rich RNA sequences from the SARS-CoV-2 genome (i.e., SCV2-RNA1 and SCV2-RNA2) activate dendritic cells. To clarify whether human neutrophils may also represent targets of SCV2-RNAs, neutrophils were treated with either SCV2-RNAs or, as a control, R848 (a TLR7/8 ligand), and were then analyzed for several functional assays and also subjected to RNA-seq experiments. Results highlight a remarkable response of neutrophils to SCV2-RNAs in terms of TNFα, IL-1ra, CXCL8 production, apoptosis delay, modulation of CD11b and CD62L expression, and release of neutrophil extracellular traps. By RNA-seq experiments, we observed that SCV2-RNA2 promotes a transcriptional reprogramming of neutrophils, characterized by the induction of thousands of proinflammatory genes, similar to that promoted by R848. Furthermore, by using CU-CPT9a, a TLR8-specific inhibitor, we found that SCV2-RNA2 stimulates neutrophils exclusively via TLR8-dependent pathways. In sum, our study proves that single-strand RNAs from the SARS-CoV-2 genome potently activate human neutrophils via TLR8, thus uncovering a potential mechanism whereby neutrophils may contribute to the pathogenesis of severe COVID-19 disease.
Keywords: COVID-19; RNA-seq; SARS-CoV-2; TLR8; neutrophil extracellular trap; neutrophils; ssRNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

