Efficacy of Clinically Used PARP Inhibitors in a Murine Model of Acute Lung Injury
- PMID: 36497049
- PMCID: PMC9738530
- DOI: 10.3390/cells11233789
Efficacy of Clinically Used PARP Inhibitors in a Murine Model of Acute Lung Injury
Abstract
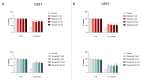
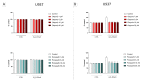
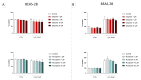
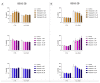
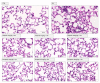
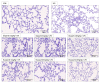
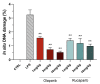
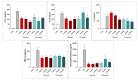
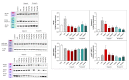
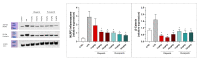
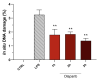
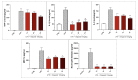
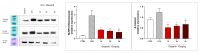
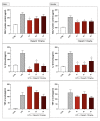
Poly(ADP-ribose) polymerase 1 (PARP1), as a potential target for the experimental therapy of acute lung injury (ALI), was identified over 20 years ago. However, clinical translation of this concept was not possible due to the lack of clinically useful PARP inhibitors. With the clinical introduction of several novel, ultrapotent PARP inhibitors, the concept of PARP inhibitor repurposing has re-emerged. Here, we evaluated the effect of 5 clinical-stage PARP inhibitors in oxidatively stressed cultured human epithelial cells and monocytes in vitro and demonstrated that all inhibitors (1-30 µM) provide a comparable degree of cytoprotection. Subsequent in vivo studies using a murine model of ALI compared the efficacy of olaparib and rucaparib. Both inhibitors (1-10 mg/kg) provided beneficial effects against lung extravasation and pro-inflammatory mediator production-both in pre- and post-treatment paradigms. The underlying mechanisms include protection against cell dysfunction/necrosis, inhibition of NF-kB and caspase 3 activation, suppression of the NLRP3 inflammasome, and the modulation of pro-inflammatory mediators. Importantly, the efficacy of PARP inhibitors was demonstrated without any potentiation of DNA damage, at least as assessed by the TUNEL method. These results support the concept that clinically approved PARP inhibitors may be repurposable for the experimental therapy of ALI.
Keywords: cell death; cytokines; inflammation; olaparib.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

