CircZXDC Promotes Vascular Smooth Muscle Cell Transdifferentiation via Regulating miRNA-125a-3p/ABCC6 in Moyamoya Disease
- PMID: 36497052
- PMCID: PMC9741004
- DOI: 10.3390/cells11233792
CircZXDC Promotes Vascular Smooth Muscle Cell Transdifferentiation via Regulating miRNA-125a-3p/ABCC6 in Moyamoya Disease
Abstract
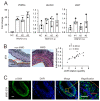
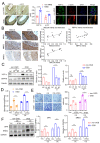
Moyamoya disease (MMD) is an occlusive, chronic cerebrovascular disease affected by genetic mutation and the immune response. Furthermore, vascular smooth muscle cells (VSMCs) and endothelial cells (ECs) participate in the neointima of MMD, but the etiology and pathophysiological changes in MMD vessels remain largely unknown. Therefore, we established the circZXDC (ZXD family zinc finger C)-miR-125a-3p-ABCC6 (ATP-binding cassette subfamily C member 6) axis from public datasets and online tools based on "sponge-like" interaction mechanisms to investigate its possible role in VSMCs. The results from a series of in vitro experiments, such as dual luciferase reporter assays, cell transfection, CCK-8 assays, Transwell assays, and Western blotting, indicate a higher level of circZXDC in the MMD plasma, especially in those MMD patients with the RNF213 mutation. Moreover, circZXDC overexpression results in a VSMC phenotype switching toward a synthetic status, with increased proliferation and migration activity. CircZXDC sponges miR-125a-3p to increase ABCC6 expression, which induces ERS (endoplasmic reticulum stress), and subsequently regulates VSMC transdifferentiation from the contractive phenotype to the synthetic phenotype, contributing to the intima thickness of MMD vessels. Our findings provide insight into the pathophysiological mechanisms of MMD and indicate that the circZXDC-miR-125a-3p-ABCC6 axis plays a pivotal role in the progression of MMD. Furthermore, circZXDC might be a diagnostic biomarker and an ABCC6-specific inhibitor and has the potential to become a promising therapeutic option for MMD.
Keywords: ABCC6; ERS; MMD; circRNA; stroke; transdifferentiation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

