Long-Term Characteristics of Human-Derived Biliary Organoids under a Single Continuous Culture Condition
- PMID: 36497057
- PMCID: PMC9741396
- DOI: 10.3390/cells11233797
Long-Term Characteristics of Human-Derived Biliary Organoids under a Single Continuous Culture Condition
Abstract
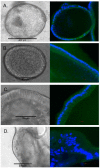
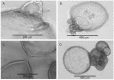
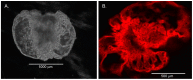
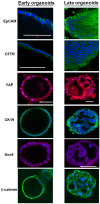
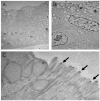
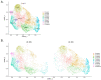
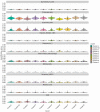
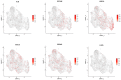
Organoids have been used to investigate the three-dimensional (3D) organization and function of their respective organs. These self-organizing 3D structures offer a distinct advantage over traditional two-dimensional (2D) culture techniques by creating a more physiologically relevant milieu to study complex biological systems. The goal of this study was to determine the feasibility of establishing organoids from various pediatric liver diseases and characterize the long-term evolution of cholangiocyte organoids (chol-orgs) under a single continuous culture condition. We established chol-orgs from 10 different liver conditions and characterized their multicellular organization into complex epithelial structures through budding, merging, and lumen formation. Immunofluorescent staining, electron microscopy, and single-nucleus RNA (snRNA-seq) sequencing confirmed the cholangiocytic nature of the chol-orgs. There were significant cell population differences in the transcript profiles of two-dimensional and organoid cultures based on snRNA-seq. Our study provides an approach for the generation and long-term maintenance of chol-orgs from various pediatric liver diseases under a single continuous culture condition.
Keywords: 3D; biliary system; extracellular matrix; microenvironment; organoid; primary cells; three dimensional.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Sampaziotis F., de Brito M.C., Madrigal P., Bertero A., Saeb-Parsy K., Soares F.A.C., Schrumpf E., Melum E., Karlsen T.H., Bradley J.A., et al. Cholangiocytes derived from human induced pluripotent stem cells for disease modeling and drug validation. Nat. Biotechnol. 2015;33:845–852. doi: 10.1038/nbt.3275. - DOI - PMC - PubMed
-
- Sampaziotis F., Muraro D., Tysoe O.C., Sawiak S., Beach T.E., Godfrey E.M., Upponi S.S., Brevini T., Wesley B.T., Garcia-Bernardo J., et al. Cholangiocyte organoids can repair bile ducts after transplantation in the human liver. Science. 2021;371:839–846. doi: 10.1126/science.aaz6964. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

