IL-5 and GM-CSF, but Not IL-3, Promote the Proliferative Properties of Inflammatory-like and Lung Resident-like Eosinophils in the Blood of Asthma Patients
- PMID: 36497064
- PMCID: PMC9740659
- DOI: 10.3390/cells11233804
IL-5 and GM-CSF, but Not IL-3, Promote the Proliferative Properties of Inflammatory-like and Lung Resident-like Eosinophils in the Blood of Asthma Patients
Abstract
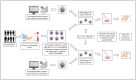
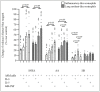
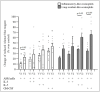
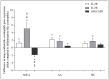
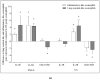
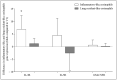
Blood eosinophils can be described as inflammatory-like (iEOS-like) and lung-resident-like (rEOS-like) eosinophils. This study is based on the hypothesis that eosinophilopoetins such as interleukin (IL)-3 and IL-5 and granulocyte-macrophage colony-stimulating factor (GM-CSF) alter the proliferative properties of eosinophil subtypes and may be associated with the expression of their receptors on eosinophils. We investigated 8 individuals with severe nonallergic eosinophilic asthma (SNEA), 17 nonsevere allergic asthma (AA), and 11 healthy subjects (HS). For AA patients, a bronchial allergen challenge with Dermatophagoides pteronyssinus was performed. Eosinophils were isolated from peripheral blood using high-density centrifugation and magnetic separation methods. The subtyping of eosinophils was based on magnetic bead-conjugated antibodies against L-selectin. Preactivation by eosinophilopoetins was performed by incubating eosinophil subtypes with IL-3, IL-5, and GM-CSF, and individual combined cell cultures were prepared with airway smooth muscle (ASM) cells. ASM cell proliferation was assessed using an Alamar blue assay. The gene expression of eosinophilopoetin receptors was analyzed with a qPCR. IL-5 and GM-CSF significantly enhanced the proliferative properties of iEOS-like and rEOS-like cells on ASM cells in both SNEA and AA groups compared with eosinophils not activated by cytokines (p < 0.05). Moreover, rEOS-like cells demonstrated a higher gene expression of the IL-3 and IL-5 receptors compared with iEOS-like cells in the SNEA and AA groups (p < 0.05). In conclusion: IL-5 and GM-CSF promote the proliferative properties of iEOS-like and rEOS-like eosinophils; however, the effect of only IL-5 may be related to the expression of its receptors in asthma patients.
Keywords: GM-CSF; IL-3; IL-5; airway smooth muscle cells; asthma; eosinophil; gene expression; proliferation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

