SAPAP Scaffold Proteins: From Synaptic Function to Neuropsychiatric Disorders
- PMID: 36497075
- PMCID: PMC9740047
- DOI: 10.3390/cells11233815
SAPAP Scaffold Proteins: From Synaptic Function to Neuropsychiatric Disorders
Abstract
Excitatory (glutamatergic) synaptic transmission underlies many aspects of brain activity and the genesis of normal human behavior. The postsynaptic scaffolding proteins SAP90/PSD-95-associated proteins (SAPAPs), which are abundant components of the postsynaptic density (PSD) at excitatory synapses, play critical roles in synaptic structure, formation, development, plasticity, and signaling. The convergence of human genetic data with recent in vitro and in vivo animal model data indicates that mutations in the genes encoding SAPAP1-4 are associated with neurological and psychiatric disorders, and that dysfunction of SAPAP scaffolding proteins may contribute to the pathogenesis of various neuropsychiatric disorders, such as schizophrenia, autism spectrum disorders, obsessive compulsive disorders, Alzheimer's disease, and bipolar disorder. Here, we review recent major genetic, epigenetic, molecular, behavioral, electrophysiological, and circuitry studies that have advanced our knowledge by clarifying the roles of SAPAP proteins at the synapses, providing new insights into the mechanistic links to neurodevelopmental and neuropsychiatric disorders.
Keywords: SAPAP/DLGAP/GKAP; animal model; cognitive dysfunction; excitatory synapse; neuropsychiatric disorders; postsynaptic scaffolding protein.
Conflict of interest statement
The authors declare no conflict of interest.
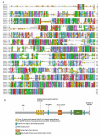
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

