Follistatin Is a Novel Chemoattractant for Migration and Invasion of Placental Trophoblasts of Mice
- PMID: 36497076
- PMCID: PMC9741044
- DOI: 10.3390/cells11233816
Follistatin Is a Novel Chemoattractant for Migration and Invasion of Placental Trophoblasts of Mice
Abstract
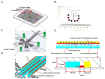
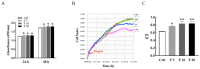
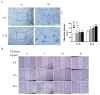
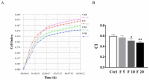
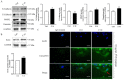
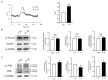
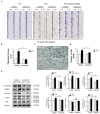
Follistatin (FST) as a gonadal protein is central to the establishment and maintenance of pregnancy. Trophoblasts' migration and invasion into the endometrium are critical events in placental development. This study aimed to elucidate the role of FST in the migration and invasion of placental trophoblasts of mice. We found that FST increased the vitality and proliferation of primary cultured trophoblasts of embryonic day 8.5 (E8.5) mice and promoted wound healing of trophoblasts. Moreover, FST significantly induced migration of trophoblasts in a microfluidic device and increased the number of invasive trophoblasts by Matrigel-coated transwell invasion assay. Being treated with FST, the adhesion of trophoblasts was inhibited, but intracellular calcium flux of trophoblasts was increased. Western blotting results showed that FST had no significant effects on the level of p-Smad3 or the ratio of p-Smad3/Smad3 in trophoblasts. Interestingly, FST elevated the level of p-JNK; the ratio of p-JNK/JNK; and expression of migration-related proteins N-cadherin, vimentin, ezrin and MMP2 in trophoblasts. Additionally, the migration of trophoblasts and expression of N-cadherin, vimentin, and MMP2 in trophoblasts induced by FST were attenuated by JNK inhibitor AS601245. These findings suggest that the elevated FST in pregnancy may act as a chemokine to induce trophoblast migration and invasion through the enhanced JNK signaling to maintain trophoblast function and promote placental development.
Keywords: JNK signaling; follistatin; migration; trophoblasts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Hardy J.T., Buhimschi I.A., McCarthy M.E., Zhao G., Laky C.A., Shook L.L., Buhimschi C.S. Imbalance of Amniotic Fluid Activin-A and Follistatin in Intraamniotic Infection, Inflammation, and Preterm Birth. J. Clin. Endocrinol. Metab. 2016;101:2785–2793. doi: 10.1210/jc.2015-4147. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

