LASP1 in Cellular Signaling and Gene Expression: More than Just a Cytoskeletal Regulator
- PMID: 36497077
- PMCID: PMC9741313
- DOI: 10.3390/cells11233817
LASP1 in Cellular Signaling and Gene Expression: More than Just a Cytoskeletal Regulator
Abstract
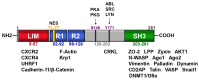
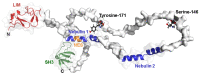
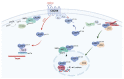
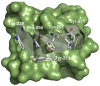
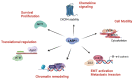
LIM and SH3 protein 1 was originally identified as a structural cytoskeletal protein with scaffolding function. However, recent data suggest additional roles in cell signaling and gene expression, especially in tumor cells. These novel functions are primarily regulated by the site-specific phosphorylation of LASP1. This review will focus on specific phosphorylation-dependent interaction between LASP1 and cellular proteins that orchestrate primary tumor progression and metastasis. More specifically, we will describe the role of LASP1 in chemokine receptor, and PI3K/AKT signaling. We outline the nuclear role for LASP1 in terms of epigenetics and transcriptional regulation and modulation of oncogenic mRNA translation. Finally, newly identified roles for the cytoskeletal function of LASP1 next to its known canonical F-actin binding properties are included.
Keywords: AKT; CXCR4; LASP1; cytoskeleton; epigenetics; nucleus; phosphorylation; structure; transcriptional regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
COPS5 and LASP1 synergistically interact to downregulate 14-3-3σ expression and promote colorectal cancer progression via activating PI3K/AKT pathway.Int J Cancer. 2018 May 1;142(9):1853-1864. doi: 10.1002/ijc.31206. Epub 2017 Dec 27. Int J Cancer. 2018. PMID: 29226323
-
LASP1 in Tumor and Tumor Microenvironment.Curr Mol Med. 2017;17(8):541-548. doi: 10.2174/1566524018666180222115103. Curr Mol Med. 2017. PMID: 29473505 Review.
-
LASP1 promotes nasopharyngeal carcinoma progression through negatively regulation of the tumor suppressor PTEN.Cell Death Dis. 2018 Mar 12;9(3):393. doi: 10.1038/s41419-018-0443-y. Cell Death Dis. 2018. PMID: 29531214 Free PMC article.
-
LIM and SH3 protein 1 induces glioma growth and invasion through PI3K/AKT signaling and epithelial-mesenchymal transition.Biomed Pharmacother. 2019 Aug;116:109013. doi: 10.1016/j.biopha.2019.109013. Epub 2019 May 27. Biomed Pharmacother. 2019. PMID: 31146105
-
An update on the LIM and SH3 domain protein 1 (LASP1): a versatile structural, signaling, and biomarker protein.Oncotarget. 2015 Jan 1;6(1):26-42. doi: 10.18632/oncotarget.3083. Oncotarget. 2015. PMID: 25622104 Free PMC article. Review.
Cited by
-
Circular RNA circSLC7A11 contributes to progression and stemness of laryngeal squamous cell carcinoma via sponging miR-877-5p from LASP1.Heliyon. 2023 Jul 14;9(7):e18290. doi: 10.1016/j.heliyon.2023.e18290. eCollection 2023 Jul. Heliyon. 2023. PMID: 37539185 Free PMC article.
-
Cord Blood Exosomal miRNAs from Small-for-Gestational-Age Newborns: Association with Measures of Postnatal Catch-Up Growth and Insulin Resistance.Int J Mol Sci. 2025 Jul 15;26(14):6770. doi: 10.3390/ijms26146770. Int J Mol Sci. 2025. PMID: 40725017 Free PMC article.
-
The Expression of Hsa-Mir-1225-5p Limits the Aggressive Biological Behaviour of Luminal Breast Cancer Cell Lines.Microrna. 2024;13(2):124-131. doi: 10.2174/0122115366268128231201054005. Microrna. 2024. PMID: 38204280 Free PMC article.
-
E7-mediated repression of miR-203 promotes LASP1-dependent proliferation in HPV-positive cervical cancer.Oncogene. 2024 Jul;43(28):2184-2198. doi: 10.1038/s41388-024-03067-4. Epub 2024 May 24. Oncogene. 2024. PMID: 38789663 Free PMC article.
-
Insights into the regulation of mRNA translation by scaffolding proteins.Biochem Soc Trans. 2024 Dec 19;52(6):2569-2578. doi: 10.1042/BST20241021. Biochem Soc Trans. 2024. PMID: 39641595 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

