The Efficacy of HGF/VEGF Gene Therapy for Limb Ischemia in Mice with Impaired Glucose Tolerance: Shift from Angiogenesis to Axonal Growth and Oxidative Potential in Skeletal Muscle
- PMID: 36497083
- PMCID: PMC9737863
- DOI: 10.3390/cells11233824
The Efficacy of HGF/VEGF Gene Therapy for Limb Ischemia in Mice with Impaired Glucose Tolerance: Shift from Angiogenesis to Axonal Growth and Oxidative Potential in Skeletal Muscle
Abstract
Background: Combined non-viral gene therapy (GT) of ischemia and cardiovascular disease is a promising tool for potential clinical translation. In previous studies our group has developed combined gene therapy by vascular endothelial growth factor 165 (VEGF165) + hepatocyte growth factor (HGF). Our recent works have demonstrated that a bicistronic pDNA that carries both human HGF and VEGF165 coding sequences has a potential for clinical application in peripheral artery disease (PAD). The present study aimed to test HGF/VEGF combined plasmid efficacy in ischemic skeletal muscle comorbid with predominant complications of PAD-impaired glucose tolerance and type 2 diabetes mellitus (T2DM).
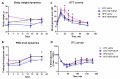
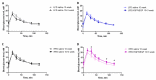
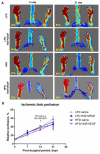
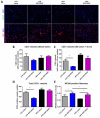
Methods: Male C57BL mice were housed on low-fat (LFD) or high-fat diet (HFD) for 10 weeks and metabolic parameters including FBG level, ITT, and GTT were evaluated. Hindlimb ischemia induction and plasmid administration were performed at 10 weeks with 3 weeks for post-surgical follow-up. Limb blood flow was assessed by laser Doppler scanning at 7, 14, and 21 days after ischemia induction. The necrotic area of m.tibialis anterior, macrophage infiltration, angio- and neuritogenesis were evaluated in tissue sections. The mitochondrial status of skeletal muscle (total mitochondria content, ETC proteins content) was assessed by Western blotting of muscle lysates.
Results: At 10 weeks, the HFD group demonstrated impaired glucose tolerance in comparison with the LFD group. HGF/VEGF plasmid injection aggravated glucose intolerance in HFD conditions. Blood flow recovery was not changed by HGF/VEGF plasmid injection either in LFD or HFD conditions. GT in LFD, but not in HFD conditions, enlarged the necrotic area and CD68+ cells infiltration. However, HGF/VEGF plasmid enhanced neuritogenesis and enlarged NF200+ area on muscle sections. In HFD conditions, HGF/VEGF plasmid injection significantly increased mitochondria content and ETC proteins content.
Conclusions: The current study demonstrated a significant role of dietary conditions in pre-clinical testing of non-viral GT drugs. HGF/VEGF combined plasmid demonstrated a novel aspect of potential participation in ischemic skeletal muscle regeneration, through regulation of innervation and bioenergetics of muscle. The obtained results made HGF/VEGF combined plasmid a very promising tool for PAD therapy in impaired glucose tolerance conditions.
Keywords: HGF; VEGF; gene therapy; high-fat diet; limb ischemia; obesity; plasmid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Combined transfer of human VEGF165 and HGF genes renders potent angiogenic effect in ischemic skeletal muscle.PLoS One. 2012;7(6):e38776. doi: 10.1371/journal.pone.0038776. Epub 2012 Jun 13. PLoS One. 2012. PMID: 22719942 Free PMC article.
-
Grain-Based Dietary Background Impairs Restoration of Blood Flow and Skeletal Muscle During Hindlimb Ischemia in Comparison With Low-Fat and High-Fat Diets.Front Nutr. 2022 Jan 10;8:809732. doi: 10.3389/fnut.2021.809732. eCollection 2021. Front Nutr. 2022. PMID: 35083264 Free PMC article.
-
Therapeutic Angiogenesis by a "Dynamic Duo": Simultaneous Expression of HGF and VEGF165 by Novel Bicistronic Plasmid Restores Blood Flow in Ischemic Skeletal Muscle.Pharmaceutics. 2020 Dec 18;12(12):1231. doi: 10.3390/pharmaceutics12121231. Pharmaceutics. 2020. PMID: 33353116 Free PMC article.
-
Gene therapy for peripheral arterial disease.Expert Opin Biol Ther. 2014 Aug;14(8):1175-84. doi: 10.1517/14712598.2014.912272. Epub 2014 Apr 28. Expert Opin Biol Ther. 2014. PMID: 24766232 Review.
-
Gene Therapy and Diabetes: A Narrative Review of Recent Advances and the Role of Multidisciplinary Healthcare Teams.Genes (Basel). 2025 Jan 20;16(1):107. doi: 10.3390/genes16010107. Genes (Basel). 2025. PMID: 39858654 Free PMC article. Review.
Cited by
-
Exerkines and myokines in aging sarcopenia.Front Endocrinol (Lausanne). 2025 Jul 29;16:1592491. doi: 10.3389/fendo.2025.1592491. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40801027 Free PMC article. Review.
-
A mechanistic computational model of HGF-VEGF-mediated endothelial cell proliferation and vascular permeability.iScience. 2025 Jul 24;28(8):113199. doi: 10.1016/j.isci.2025.113199. eCollection 2025 Aug 15. iScience. 2025. PMID: 40822341 Free PMC article.
-
Regenerative rehabilitation measures to restore tissue function after arsenic exposure.Curr Opin Biomed Eng. 2024 Jun;30:100529. doi: 10.1016/j.cobme.2024.100529. Epub 2024 Mar 11. Curr Opin Biomed Eng. 2024. PMID: 40191583 Free PMC article.
-
Gene Therapeutic Drug pCMV-VEGF165 Plasmid ('Neovasculgen') Promotes Gingiva Soft Tissue Augmentation in Rabbits.Int J Mol Sci. 2024 Sep 17;25(18):10013. doi: 10.3390/ijms251810013. Int J Mol Sci. 2024. PMID: 39337502 Free PMC article.
-
Diabetes mellitus in peripheral artery disease: Beyond a risk factor.Front Cardiovasc Med. 2023 Apr 17;10:1148040. doi: 10.3389/fcvm.2023.1148040. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37139134 Free PMC article. Review.
References
-
- Kastrup J., Jørgensen E., Rück A., Tägil K., Glogar D., Ruzyllo W., Bøtker H.E., Dudek D., Drvota V., Hesse B., et al. Direct intramyocardial plasmid vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris A randomized double-blind placebo-controlled study: The Euroinject One trial. J. Am. Coll. Cardiol. 2005;45:982–988. doi: 10.1016/j.jacc.2004.12.068. - DOI - PubMed
-
- Penn M.S., Mendelsohn F.O., Schaer G.L., Sherman W., Farr M. An open-label dose escalation study to evaluate the safety of administration of nonviral stromal cell-derived factor-1 plasmid to treat symptomatic ischemic heart failure. Circ. Res. 2013;112:816–825. doi: 10.1161/CIRCRESAHA.111.300440. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

