Hypothalamic-Pituitary-Adrenal Hormones Impair Pig Fertilization and Preimplantation Embryo Development via Inducing Oviductal Epithelial Apoptosis: An In Vitro Study
- PMID: 36497149
- PMCID: PMC9740987
- DOI: 10.3390/cells11233891
Hypothalamic-Pituitary-Adrenal Hormones Impair Pig Fertilization and Preimplantation Embryo Development via Inducing Oviductal Epithelial Apoptosis: An In Vitro Study
Abstract
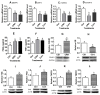
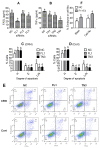
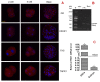
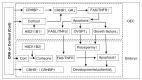
Previous studies show that stressful events after ovulation in sows significantly impaired the embryo cleavage with a significant elevation of blood cortisol. However, the effects of corticotropin-releasing hormone (CRH), adrenocorticotropic hormone (ACTH) and cortisol on fertilization and embryo development remain to be specified, and whether they damage pig embryos directly or indirectly is unclear. This study demonstrated that embryo development was unaffected when pig parthenotes were cultured with different concentrations of CRH/ACTH/cortisol. However, embryo development was significantly impaired when the embryos were cocultured with pig oviductal epithelial cells (OECs) in the presence of CRH/cortisol or cultured in medium that was conditioned with CRH/cortisol-pretreated OECs (CRH/cortisol-CM). Fertilization in CRH/cortisol-CM significantly increased the rates of polyspermy. CRH and cortisol induced apoptosis of OECs through FAS and TNFα signaling. The apoptotic OECs produced less growth factors but more FASL and TNFα, which induced apoptosis in embryos. Pig embryos were not sensitive to CRH because they expressed no CRH receptor but the CRH-binding protein, and they were tolerant to cortisol because they expressed more 11-beta hydroxysteroid dehydrogenase 2 (HSD11B2) than HSD11B1. When used at a stress-induced physiological concentration, while culture with either CRH or cortisol alone showed no effect, culture with both significantly increased apoptosis in OECs. In conclusion, CRH and cortisol impair pig fertilization and preimplantation embryo development indirectly by inducing OEC apoptosis via the activation of the FAS and TNFα systems. ACTH did not show any detrimental effect on pig embryos, nor OECs.
Keywords: HPA hormones; apoptosis; embryo development; oviduct; pig.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Corticotrophin-releasing hormone and corticosterone impair development of preimplantation embryos by inducing oviductal cell apoptosis via activating the Fas system: an in vitro study.Hum Reprod. 2017 Aug 1;32(8):1583-1597. doi: 10.1093/humrep/dex217. Hum Reprod. 2017. PMID: 28591825
-
Preimplantation maternal stress impairs embryo development by inducing oviductal apoptosis with activation of the Fas system.Mol Hum Reprod. 2016 Nov;22(11):778-790. doi: 10.1093/molehr/gaw052. Epub 2016 Jul 29. Mol Hum Reprod. 2016. PMID: 27475493
-
Corticosterone induced apoptosis of mouse oviduct epithelial cells independent of the TNF-α system.J Reprod Dev. 2021 Feb 15;67(1):43-51. doi: 10.1262/jrd.2020-122. Epub 2020 Dec 12. J Reprod Dev. 2021. PMID: 33310974 Free PMC article.
-
Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum.Ann N Y Acad Sci. 2003 Nov;997:136-49. doi: 10.1196/annals.1290.016. Ann N Y Acad Sci. 2003. PMID: 14644820 Review.
-
Sleep and the hypothalamo-pituitary-adrenocortical system.Sleep Med Rev. 2002 Apr;6(2):125-38. doi: 10.1053/smrv.2001.0159. Sleep Med Rev. 2002. PMID: 12531148 Review.
Cited by
-
Role and action mechanisms of tPA in CRH-induced apoptosis of mouse oviductal epithelial and mural granulosa cells.J Reprod Dev. 2024 Aug 7;70(4):238-246. doi: 10.1262/jrd.2024-028. Epub 2024 Jun 24. J Reprod Dev. 2024. PMID: 38910127 Free PMC article.
-
Maternal stress and the early embryonic microenvironment: investigating long-term cortisol effects on bovine oviductal epithelial cells using air-liquid interface culture.J Anim Sci Biotechnol. 2024 Oct 3;15(1):129. doi: 10.1186/s40104-024-01087-4. J Anim Sci Biotechnol. 2024. PMID: 39358766 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

