Advances in Cellular Reprogramming-Based Approaches for Heart Regenerative Repair
- PMID: 36497171
- PMCID: PMC9740402
- DOI: 10.3390/cells11233914
Advances in Cellular Reprogramming-Based Approaches for Heart Regenerative Repair
Abstract
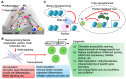
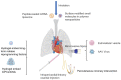
Continuous loss of cardiomyocytes (CMs) is one of the fundamental characteristics of many heart diseases, which eventually can lead to heart failure. Due to the limited proliferation ability of human adult CMs, treatment efficacy has been limited in terms of fully repairing damaged hearts. It has been shown that cell lineage conversion can be achieved by using cell reprogramming approaches, including human induced pluripotent stem cells (hiPSCs), providing a promising therapeutic for regenerative heart medicine. Recent studies using advanced cellular reprogramming-based techniques have also contributed some new strategies for regenerative heart repair. In this review, hiPSC-derived cell therapeutic methods are introduced, and the clinical setting challenges (maturation, engraftment, immune response, scalability, and tumorigenicity), with potential solutions, are discussed. Inspired by the iPSC reprogramming, the approaches of direct cell lineage conversion are merging, such as induced cardiomyocyte-like cells (iCMs) and induced cardiac progenitor cells (iCPCs) derived from fibroblasts, without induction of pluripotency. The studies of cellular and molecular pathways also reveal that epigenetic resetting is the essential mechanism of reprogramming and lineage conversion. Therefore, CRISPR techniques that can be repurposed for genomic or epigenetic editing become attractive approaches for cellular reprogramming. In addition, viral and non-viral delivery strategies that are utilized to achieve CM reprogramming will be introduced, and the therapeutic effects of iCMs or iCPCs on myocardial infarction will be compared. After the improvement of reprogramming efficiency by developing new techniques, reprogrammed iCPCs or iCMs will provide an alternative to hiPSC-based approaches for regenerative heart therapies, heart disease modeling, and new drug screening.
Keywords: direct reprogramming; engineered heart tissue; iPSC-CMs; immune reduction; myocadiac infarction; progenitor cells; regenerative heart repair; stem cells.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Virani S.S., Alonso A., Aparicio H.J., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Cheng S., Delling F.N., et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950. - DOI - PubMed
-
- Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M., Barengo N.C., Beaton A.Z., Benjamin E.J., Benziger C.P., et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. J. Am. Coll. Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

