Perineural Invasion in Pancreatic Ductal Adenocarcinoma: From Molecules towards Drugs of Clinical Relevance
- PMID: 36497277
- PMCID: PMC9739544
- DOI: 10.3390/cancers14235793
Perineural Invasion in Pancreatic Ductal Adenocarcinoma: From Molecules towards Drugs of Clinical Relevance
Abstract
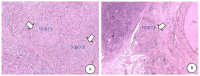
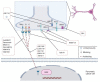
Pancreatic ductal adenocarcinoma is one of the most threatening solid malignancies. Molecular and cellular mediators that activate paracrine signalling also regulate the dynamic interaction between pancreatic cancer cells and nerves. This reciprocal interface leads to perineural invasion (PNI), defined as the ability of cancer cells to invade nerves, similar to vascular and lymphatic metastatic cascade. Targeting PNI in pancreatic cancer might help ameliorate prognosis and pain relief. In this review, the modern knowledge of PNI in pancreatic cancer has been analysed and critically presented. We focused on molecular pathways promoting cancer progression, with particular emphasis on neuropathic pain generation, and we reviewed the current knowledge of pharmacological inhibitors of the PNI axis. PNI represents a common hallmark of PDAC and correlates with recurrence, poor prognosis and pain in pancreatic cancer patients. The interaction among pancreatic cancer cells, immune cells and nerves is biologically relevant in each stage of the disease and stimulates great interest, but the real impact of the administration of novel agents in clinical practice is limited. It is still early days for PNI-targeted treatments, and further advanced studies are needed to understand whether they could be effective tools in the clinical setting.
Keywords: neuropathic pain; pancreas; pancreatic ductal adenocarcinoma; perineural invasion; tumour microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Gourgou-Bourgade S., Bascoul-Mollevi C., Desseigne F., Ychou M., Bouché O., Guimbaud R., Bécouarn Y., Adenis A., Raoul J.L., Boige V., et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: Results from the PRODIGE 4/ACCORD 11 randomized trial. J. Clin. Oncol. 2013;31:23–29. doi: 10.1200/JCO.2012.44.4869. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

