Zebrafish as an Orthotopic Tumor Model for Retinoblastoma Mimicking Routes of Human Metastasis
- PMID: 36497295
- PMCID: PMC9736091
- DOI: 10.3390/cancers14235814
Zebrafish as an Orthotopic Tumor Model for Retinoblastoma Mimicking Routes of Human Metastasis
Abstract
Background: Retinoblastoma (RB) is the most common eye cancer in children that has a high mortality rate when left untreated. Mouse models for retinoblastoma have been established but are time- and cost-intensive. The aim of this work was to evaluate an orthotopic transplantation model of retinoblastoma in zebrafish that also allows for tracking migratory routes and to explore advantages and disadvantages with respect to drug testing.
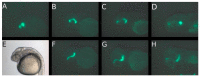
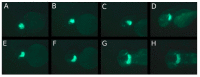
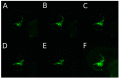
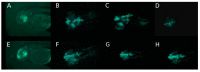
Methods: Three fluorescence-labeled retinoblastoma cell lines (RB355, WERI-RB-1, Y79) were injected into the left eye of two-day-old zebrafish, while the un-injected right eye served as control. The migratory trajectories of injected retinoblastoma cells were observed until 8 days post injection (dpi), both in lateral and dorsal view, and measuring fluorescence intensity of injected cells was done for RB355 cells.
Results: Time until the onset of migration and routes for all three retinoblastoma cell lines were comparable and resulted in migration into the brain and ventricles of the forebrain, midbrain and hindbrain. Involvement of the optic nerve was observed in 10% of injections with the RB355 cell line, 15% with Y79 cells and 5% with WERI-RB-1 cells. Fluorescence intensity of injected RB355 cells showed an initial increase until five dpi, but then decreased with high variability until the end of observation.
Conclusion: The zebrafish eye is well suited for the analysis of migratory routes in retinoblastoma and closely mirrors patterns of retinoblastoma metastases in humans.
Keywords: RB355; WERI-RB-1; Y79; childhood eye cancer; malignant retinal tumor; orthotopic transplantation; retinoblastoma; zebrafish.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Retinoblastoma cell lines Y79, RB355 and WERI-Rb27 are genetically related.Ophthalmic Paediatr Genet. 1991 Mar;12(1):49-56. doi: 10.3109/13816819109023085. Ophthalmic Paediatr Genet. 1991. PMID: 1679230
-
Expression Changes and Impact of the Extracellular Matrix on Etoposide Resistant Human Retinoblastoma Cell Lines.Int J Mol Sci. 2020 Jun 17;21(12):4322. doi: 10.3390/ijms21124322. Int J Mol Sci. 2020. PMID: 32560557 Free PMC article.
-
Metastatic and nonmetastatic models of retinoblastoma.Am J Pathol. 2000 Oct;157(4):1405-12. doi: 10.1016/S0002-9440(10)64653-6. Am J Pathol. 2000. PMID: 11021842 Free PMC article.
-
Chemosensitization to adriamycin by cyclosporin A and verapamil in human retinoblastoma cell lines.J Korean Med Sci. 1993 Apr;8(2):104-9. doi: 10.3346/jkms.1993.8.2.104. J Korean Med Sci. 1993. PMID: 8397925 Free PMC article.
-
Newly established human retinoblastoma cell lines exhibit an "immortalized" but not an invasive phenotype in vitro.Int J Cancer. 1990 Jul 15;46(1):125-32. doi: 10.1002/ijc.2910460123. Int J Cancer. 1990. PMID: 2365495
Cited by
-
Targeting DNA Topoisomerase IIα in Retinoblastoma: Implications in EMT and Therapeutic Strategies.Biologics. 2025 Mar 18;19:113-123. doi: 10.2147/BTT.S499314. eCollection 2025. Biologics. 2025. PMID: 40123578 Free PMC article.
-
Oxidative Stress, Genetic Factors and Behavioral Responses to Chemical Exposure: Insights into Cancer Development in Zebrafish (Danio Rerio).Biochem Genet. 2025 Jun 3. doi: 10.1007/s10528-025-11148-6. Online ahead of print. Biochem Genet. 2025. PMID: 40459645 Review.
-
Histone Deacetylases in Retinoblastoma.Int J Mol Sci. 2024 Jun 24;25(13):6910. doi: 10.3390/ijms25136910. Int J Mol Sci. 2024. PMID: 39000021 Free PMC article. Review.
References
-
- Wong E.S., Choy R.W., Zhang Y., Chu W.K., Chen L.J., Pang C.P., Yam J.C. Global Retinoblastoma Survival and Globe Preservation: A Systematic Review and Meta-Analysis of Associations with Socioeconomic and Health-Care Factors. Lancet Glob. Health. 2022;10:E380–E389. doi: 10.1016/S2214-109X(21)00555-6. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

