The Chick Embryo Xenograft Model for Malignant Pleural Mesothelioma: A Cost and Time Efficient 3Rs Model for Drug Target Evaluation
- PMID: 36497318
- PMCID: PMC9740959
- DOI: 10.3390/cancers14235836
The Chick Embryo Xenograft Model for Malignant Pleural Mesothelioma: A Cost and Time Efficient 3Rs Model for Drug Target Evaluation
Abstract
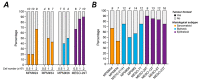
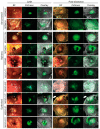
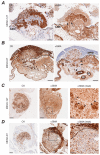
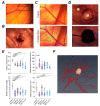
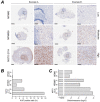
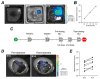
Malignant pleural mesothelioma (MPM) has limited treatment options and poor prognosis. Frequent inactivation of the tumour suppressors BAP1, NF2 and P16 may differentially sensitise tumours to treatments. We have established chick chorioallantoic membrane (CAM) xenograft models of low-passage MPM cell lines and protocols for evaluating drug responses. Ten cell lines, representing the spectrum of histological subtypes and tumour suppressor status, were dual labelled for fluorescence/bioluminescence imaging and implanted on the CAM at E7. Bioluminescence was used to assess viability of primary tumours, which were excised at E14 for immunohistological staining or real-time PCR. All MPM cell lines engrafted efficiently forming vascularised nodules, however their size, morphology and interaction with chick cells varied. MPM phenotypes including local invasion, fibroblast recruitment, tumour angiogenesis and vascular remodelling were evident. Bioluminescence imaging could be used to reliably estimate tumour burden pre- and post-treatment, correlating with tumour weight and Ki-67 staining. In conclusion, MPM-CAM models recapitulate important features of the disease and are suitable to assess drug targets using a broad range of MPM cell lines that allow histological or genetic stratification. They are amenable to multi-modal imaging, potentially offering a time and cost-efficient, 3Rs-compliant alternative to rodent xenograft models to prioritise candidate compounds from in vitro studies.
Keywords: 3Rs; CAM; MRI; bioluminescence; chick embryo; fluorescence; histology; mesothelioma; preclinical; xenograft.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Blum Y., Meiller C., Quetel L., Elarouci N., Ayadi M., Tashtanbaeva D., Armenoult L., Montagne F., Tranchant R., Renier A., et al. Dissecting heterogeneity in malignant pleural mesothelioma through histo-molecular gradients for clinical applications. Nat. Commun. 2019;10:1333. doi: 10.1038/s41467-019-09307-6. - DOI - PMC - PubMed
-
- Galateau-Salle F., Churg A., Roggli V., Travis W.D., World Health Organization Committee for Tumors of the Pleura The 2015 World Health Organization Classification of Tumors of the Pleura: Advances since the 2004 Classification. J. Thorac. Oncol. 2016;11:142–154. doi: 10.1016/j.jtho.2015.11.005. - DOI - PubMed
-
- Ohara Y., Enomoto A., Tsuyuki Y., Sato K., Iida T., Kobayashi H., Mizutani Y., Miyai Y., Hara A., Mii S., et al. Connective tissue growth factor produced by cancer-associated fibroblasts correlates with poor prognosis in epithelioid malignant pleural mesothelioma. Oncol. Rep. 2020;44:838–848. doi: 10.3892/or.2020.7669. - DOI - PMC - PubMed
-
- Li Q., Wang W., Yamada T., Matsumoto K., Sakai K., Bando Y., Uehara H., Nishioka Y., Sone S., Iwakiri S., et al. Pleural mesothelioma instigates tumor-associated fibroblasts to promote progression via a malignant cytokine network. Am. J. Pathol. 2011;179:1483–1493. doi: 10.1016/j.ajpath.2011.05.060. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

