Insights into the Role of Sialylation in Cancer Metastasis, Immunity, and Therapeutic Opportunity
- PMID: 36497322
- PMCID: PMC9737300
- DOI: 10.3390/cancers14235840
Insights into the Role of Sialylation in Cancer Metastasis, Immunity, and Therapeutic Opportunity
Abstract
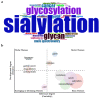
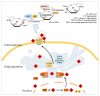
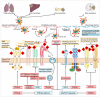
Sialylation is an enzymatic process that covalently attaches sialic acids to glycoproteins and glycolipids and terminates them by creating sialic acid-containing glycans (sialoglycans). Sialoglycans, usually located in the outmost layers of cells, play crucial biological roles, notably in tumor transformation, growth, metastasis, and immune evasion. Thus, a deeper comprehension of sialylation in cancer will help to facilitate the development of innovative cancer therapies. Cancer sialylation-related articles have consistently increased over the last four years. The primary subjects of these studies are sialylation, cancer, immunotherapy, and metastasis. Tumor cells activate endothelial cells and metastasize to distant organs in part by the interactions of abnormally sialylated integrins with selectins. Furthermore, cancer sialylation masks tumor antigenic epitopes and induces an immunosuppressive environment, allowing cancer cells to escape immune monitoring. Cytotoxic T lymphocytes develop different recognition epitopes for glycosylated and nonglycosylated peptides. Therefore, targeting tumor-derived sialoglycans is a promising approach to cancer treatments for limiting the dissemination of tumor cells, revealing immunogenic tumor antigens, and boosting anti-cancer immunity. Exploring the exact tumor sialoglycans may facilitate the identification of new glycan targets, paving the way for the development of customized cancer treatments.
Keywords: anti-tumor therapy; cancer; immunotherapy; metastasis; sialic acid; sialylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Macbeth R.A.L., Bekesi J.G. Plasma glycoproteins in various disease states including carcinoma. Cancer Res. 1962;22:1170–1176.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

