The Study of the Extracellular Matrix in Chronic Inflammation: A Way to Prevent Cancer Initiation?
- PMID: 36497384
- PMCID: PMC9741172
- DOI: 10.3390/cancers14235903
The Study of the Extracellular Matrix in Chronic Inflammation: A Way to Prevent Cancer Initiation?
Abstract
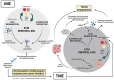
Bidirectional communication between cells and their microenvironment has a key function in normal tissue homeostasis, and in disease initiation, progression and a patient's prognosis, at the very least. The extracellular matrix (ECM), as an element of all tissues and cellular microenvironment, is a frequently overlooked component implicated in the pathogenesis and progression of several diseases. In the inflammatory microenvironment (IME), different alterations resulting from remodeling processes can affect ECM, progressively inducing cancer initiation and the passage toward a tumor microenvironment (TME). Indeed, it has been demonstrated that altered ECM components interact with a variety of surface receptors triggering intracellular signaling that affect cellular pathways in turn. This review aims to support the notion that the ECM and its alterations actively participate in the promotion of chronic inflammation and cancer initiation. In conclusion, some data obtained in cancer research with the employment of decellularized ECM (dECM) models are described. The reported results encourage the application of dECM models to investigate the short circuits contributing to the creation of distinct IME, thus representing a potential tool to avoid the progression toward a malignant lesion.
Keywords: 3D culture model; cancer; chronic inflammation; decellularization; extracellular matrix.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Decellularized Extracellular Matrix for Cancer Research.Materials (Basel). 2019 Apr 22;12(8):1311. doi: 10.3390/ma12081311. Materials (Basel). 2019. PMID: 31013621 Free PMC article. Review.
-
Decellularized heart ECM hydrogel using supercritical carbon dioxide for improved angiogenesis.Acta Biomater. 2018 Feb;67:270-281. doi: 10.1016/j.actbio.2017.11.046. Epub 2017 Dec 6. Acta Biomater. 2018. PMID: 29223704
-
Mesenchymal Stromal Cell-Produced Components of Extracellular Matrix Potentiate Multipotent Stem Cell Response to Differentiation Stimuli.Front Cell Dev Biol. 2020 Sep 22;8:555378. doi: 10.3389/fcell.2020.555378. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33072743 Free PMC article.
-
Inflammation, Extracellular Matrix Remodeling, and Proteostasis in Tumor Microenvironment.Int J Mol Sci. 2021 Jul 28;22(15):8102. doi: 10.3390/ijms22158102. Int J Mol Sci. 2021. PMID: 34360868 Free PMC article. Review.
-
Decellularized Extracellular Matrix for Cell Biology.Curr Protoc. 2021 Dec;1(12):e318. doi: 10.1002/cpz1.318. Curr Protoc. 2021. PMID: 34878719
Cited by
-
Modelling Cancer Pathophysiology: Mechanisms and Changes in the Extracellular Matrix During Cancer Initiation and Early Tumour Growth.Cancers (Basel). 2025 May 15;17(10):1675. doi: 10.3390/cancers17101675. Cancers (Basel). 2025. PMID: 40427172 Free PMC article. Review.
-
[Xinfeng Capsule alleviates interleukin-1β-induced chondrocyte inflammation and extracellular matrix degradation by regulating the miR-502-5p/TRAF2/NF-κB axis].Nan Fang Yi Ke Da Xue Xue Bao. 2024 Jan 20;44(1):108-118. doi: 10.12122/j.issn.1673-4254.2024.01.13. Nan Fang Yi Ke Da Xue Xue Bao. 2024. PMID: 38293982 Free PMC article. Chinese.
-
Expression of Stemness Markers in the Cervical Smear of Patients with Cervical Insufficiency.Cells. 2023 Apr 18;12(8):1183. doi: 10.3390/cells12081183. Cells. 2023. PMID: 37190092 Free PMC article.
-
Simulation of the Diffusion Characteristics of Multifunctional Nanocarriers in Tumor Tissues Using Lattice Gas Automata and the Lattice Boltzmann Method.Bioengineering (Basel). 2025 Apr 18;12(4):429. doi: 10.3390/bioengineering12040429. Bioengineering (Basel). 2025. PMID: 40281789 Free PMC article.
-
The Role of Pericytes in Inner Ear Disorders: A Comprehensive Review.Biology (Basel). 2024 Oct 8;13(10):802. doi: 10.3390/biology13100802. Biology (Basel). 2024. PMID: 39452111 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

