Adrenomedullin Secreted by Melanoma Cells Promotes Melanoma Tumor Growth through Angiogenesis and Lymphangiogenesis
- PMID: 36497391
- PMCID: PMC9738606
- DOI: 10.3390/cancers14235909
Adrenomedullin Secreted by Melanoma Cells Promotes Melanoma Tumor Growth through Angiogenesis and Lymphangiogenesis
Abstract
Introduction: Metastatic melanoma is an aggressive tumor and can constitute a real therapeutic challenge despite the significant progress achieved with targeted therapies and immunotherapies, thus highlighting the need for the identification of new therapeutic targets. Adrenomedullin (AM) is a peptide with significant expression in multiple types of tumors and is multifunctional. AM impacts angiogenesis and tumor growth and binds to calcitonin receptor-like receptor/receptor activity-modifying protein 2 or 3 (CLR/RAMP2; CLR/RAMP3).
Methods: In vitro and in vivo studies were performed to determine the functional role of AM in melanoma growth and tumor-associated angiogenesis and lymphangiogenesis.
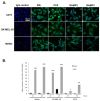
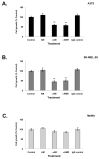
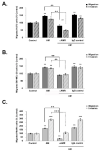
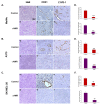
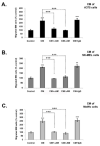
Results: In this study, AM and AM receptors were immunohistochemically localized in the tumoral compartment of melanoma tissue, suggesting that the AM system plays a role in melanoma growth. We used A375, SK-MEL-28, and MeWo cells, for which we demonstrate an expression of AM and its receptors; hypoxia induces the expression of AM in melanoma cells. The proliferation of A375 and SK-MEL-28 cells is decreased by anti-AM antibody (αAM) and anti-AMR antibodies (αAMR), supporting the fact that AM may function as a potent autocrine/paracrine growth factor for melanoma cells. Furthermore, migration and invasion of melanoma cells increased after treatment with AM and decreased after treatment with αAMR, thus indicating that melanoma cells are regulated by AM. Systemic administration of αAMR reduced neovascularization of in vivo Matrigel plugs containing melanoma cells, as demonstrated by reduced numbers of vessel structures, which suggests that AM is one of the melanoma cells-derived factors responsible for endothelial cell-like and pericyte recruitment in the construction of neovascularization. In vivo, αAMR therapy blocked angiogenesis and lymphangiogenesis and decreased proliferation in MeWo xenografts, thereby resulting in tumor regression. Histological examination of αAMR-treated tumors showed evidence of the disruption of tumor vascularity, with depletion of vascular endothelial cells and a significant decrease in lymphatic endothelial cells.
Conclusions: The expression of AM by melanoma cells promotes tumor growth and neovascularization by supplying/amplifying signals for neoangiogenesis and lymphangiogenesis.
Keywords: A375; MeWo; SK-MEL-28; adrenomedullin; angiogenesis; invasion; lymphangiogenesis; melanoma; tumor growth.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Greig R., Dunnington D., Murthy U., Anzano M. Growth Factors as Novel Therapeutic Targets in Neoplastic Disease. Cancer Surv. 1988;7:653–674. - PubMed
-
- Fernandez-Sauze S., Delfino C., Mabrouk K., Dussert C., Chinot O., Martin P.-M., Grisoli F., Ouafik L. Effects of adrenomedullin on endothelial cells in the multistep process of angiogenesis: Involvement of CRLR/RAMP2 and CRLR/RAMP3 receptors. Int. J. Cancer. 2003;108:797–804. doi: 10.1002/ijc.11663. - DOI - PubMed
LinkOut - more resources
Full Text Sources

