Inhibition of Vascular Endothelial Growth Factor Protects against the Development of Oxaliplatin-Induced Sinusoidal Obstruction Syndrome in Wild-Type but Not in CD39-Null Mice
- PMID: 36497474
- PMCID: PMC9739893
- DOI: 10.3390/cancers14235992
Inhibition of Vascular Endothelial Growth Factor Protects against the Development of Oxaliplatin-Induced Sinusoidal Obstruction Syndrome in Wild-Type but Not in CD39-Null Mice
Abstract
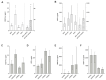
(1) Background: Sinusoidal obstruction syndrome (SOS) after oxaliplatin-based chemotherapy is associated with unfavorable outcomes after partial hepatectomy for colorectal liver metastases (CLM). Bevacizumab, a monoclonal antibody against vascular endothelial growth factor (VEGF), may prevent SOS development. We investigated the impact of VEGF-inhibition on the development of SOS in a murine model. (2) Methods: Male wild-type and CD39-null mice received oxaliplatin, additional anti-VEGF (OxAV), or controls, and were sacrificed or subjected to major partial hepatectomy (MH). Specimen were used for histological analysis of SOS. Liver damage was assessed by plasma transaminases. The VEGF pathway was elucidated by quantitative PCR of liver tissue and protein analysis of plasma. (3) Results: Mice treated with oxaliplatin developed SOS. Concomitant anti-VEGF facilitated a reduced incidence of SOS, but not in CD39-null mice. SOS was associated with increased plasma VEGF-A and decreased hepatocyte growth factor (HGF). After OxAV treatment, VEGF-R2 was upregulated in wild-type but downregulated in CD39-null mice. Oxaliplatin alone was associated with higher liver damage after MH than in mice with concomitant VEGF-inhibition. (4) Conclusions: We established a murine model of oxaliplatin-induced SOS and provided novel evidence on the protective effect of VEGF-inhibition against the development of SOS that may be associated with changes in the pathway of VEGF and its receptor VEGF-R2.
Keywords: bevacizumab; colorectal liver metastases; sinusoidal obstruction syndrome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Van Cutsem E., Cervantes A., Adam R., Sobrero A., van Krieken J.H., Aderka D., Aguilar E.A., Bardelli A., Benson A., Bodoky G., et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. - DOI - PubMed
-
- Rubbia-Brandt L., Audard V., Sartoretti P., Roth A.D., Brezault C., Le Charpentier M., Dousset B., Morel P., Soubrane O., Chaussade S., et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann. Oncol. 2004;15:460–466. doi: 10.1093/annonc/mdh095. - DOI - PubMed
-
- Duwe G., Knitter S., Pesthy S., Beierle A.S., Bahra M., Schmelzle M., Schmuck R.B., Lohneis P., Raschzok N., Öllinger R., et al. Hepatotoxicity following systemic therapy for colorectal liver metastases and the impact of chemotherapy-associated liver injury on outcomes after curative liver resection. Eur. J. Surg. Oncol. 2017;43:1668–1681. doi: 10.1016/j.ejso.2017.05.008. - DOI - PubMed
-
- Aloia T., Sebagh M., Plasse M., Karam V., Lévi F., Giacchetti S., Azoulay D., Bismuth H., Castaing D., Adam R. Liver Histology and Surgical Outcomes After Preoperative Chemotherapy with Fluorouracil Plus Oxaliplatin in Colorectal Cancer Liver Metastases. J. Clin. Oncol. 2006;24:4983–4990. doi: 10.1200/JCO.2006.05.8156. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

