The Reporting, Use, and Validity of Patient-Reported Outcomes in Multiple Myeloma in Clinical Trials: A Systematic Literature Review
- PMID: 36497488
- PMCID: PMC9741479
- DOI: 10.3390/cancers14236007
The Reporting, Use, and Validity of Patient-Reported Outcomes in Multiple Myeloma in Clinical Trials: A Systematic Literature Review
Abstract
Background: Patient-reported outcomes (PROs) are becoming increasingly important in supporting clinical outcomes in clinical trials. In multiple myeloma (MM), PRO measurement is useful to reveal how treatment affects physical, psychosocial, and functional behaviour as well as symptoms and treatment-related adverse events to evaluate the benefit-risk ratio of a particular drug or drug combination. We report the types of PRO instruments used in MM, the frequency in which they are utilised in randomised controlled trials (RCTs), and the consistency of their reporting.
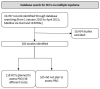
Methods: The European Hematology Association (EHA) supports the development of guidelines for the use of PROs in adult patients with haematological malignancies. The first step is the present systematic review of the literature. MEDLINE and CENTRAL were searched for RCTs in MM between 2015 and 2020. Study design, characteristics of MM and its treatment, the primary outcomes, and the types of PRO instrument(s) were extracted using a predefined template. Additionally, in a stepwise approach, it was assessed whether the identified instruments had been validated for multiple myeloma patients, patients with haematological malignancies, or cancer patients.
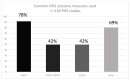
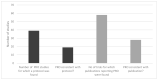
Results: Following screening for RCTs, 283 studies were included for review from 10,707 records retrieved, and 118 of these planned the use of PRO measures. Thirty-eight PRO instruments were reported. The most frequently used instrument (92 studies) was the EORTC QLQ-30. The EORTC-MY20 MM-specific questionnaire was the second most frequently used (50 studies), together with the EQ-5D (50 studies). Only 19 PRO instruments reported were consistent with the trial registry. Furthermore, in 58 publications, the information on PRO instruments differed between the publication and the trial registry. Further, information on PRO in HTA reports was available for 26 studies, of which 18 reports were consistent with the trial registries. Out of the 38 instruments used, six had been validated for patients with multiple myeloma (the most frequently used), six for patients with haematological malignancies, and 10 for cancer patients in general.
Conclusions: The findings indicate that the measurement of PROs in RCTs for MM is underutilised, underreported, and often inconsistent. Guidelines for the appropriate use of PROs in MM are needed to ensure standardisation in selection and reporting. Furthermore, not all PRO instruments identified have been validated for myeloma patients or patients with haematological malignancies. Thus, guidelines for the appropriate use and reporting of PROs are needed in MM to ensure standardisation in the selection and reporting of PROs.
Keywords: clinical trials; multiple myeloma; patient-reported outcomes; quality of life; symptoms; systematic review; validation studies.
Conflict of interest statement
S.S. has received an honorarium for providing consultancy to NovoNordisk and Abbvie and is a copyright holder of several instruments, including FROM-16, MLCDP, T-QoL, HidroQoL, PDF, RQLP, and the HM-PRO. E.N.O. has received an honorarium for providing consultancy and participating in speakers’ panels for AbbVie, Alexion, Amgen, Janssen, Daiichi, and Novartis. She is a copyright holder of the QOL-E instrument and the HM-PRO. T.I. is the Principal Investigator of sponsor-initiated observational studies for Amgen and Takeda, has received an honorarium for participating in speakers’ panels for BMS, Amgen, and Novartis and is a copyright holder of the HM-PRO. E.L., N.S., N.K., M.A.: none known.
Figures
Similar articles
-
Patient-reported outcomes in Hodgkin lymphoma trials: a systematic review.Front Oncol. 2024 Mar 13;14:1353101. doi: 10.3389/fonc.2024.1353101. eCollection 2024. Front Oncol. 2024. PMID: 38544841 Free PMC article.
-
Quality of patient-reported outcome reporting in randomised controlled trials of haematological malignancies according to international quality standards: a systematic review.Lancet Haematol. 2020 Dec;7(12):e892-e901. doi: 10.1016/S2352-3026(20)30292-1. Lancet Haematol. 2020. PMID: 33242446
-
Health-related quality of life in patients with triple-class exposed relapsed and refractory multiple myeloma treated with idecabtagene vicleucel or standard regimens: patient-reported outcomes from the phase 3, randomised, open-label KarMMa-3 clinical trial.Lancet Haematol. 2024 Mar;11(3):e216-e227. doi: 10.1016/S2352-3026(24)00005-X. Lancet Haematol. 2024. PMID: 38423700 Clinical Trial.
-
Patient-Reported Outcomes in Phase 3 Clinical Trials for Blood Cancers: A Systematic Review.JAMA Netw Open. 2024 Jun 3;7(6):e2414425. doi: 10.1001/jamanetworkopen.2024.14425. JAMA Netw Open. 2024. PMID: 38829615 Free PMC article.
-
Informative value of Patient Reported Outcomes (PRO) in Health Technology Assessment (HTA).GMS Health Technol Assess. 2011 Feb 2;7:Doc01. doi: 10.3205/hta000092. GMS Health Technol Assess. 2011. PMID: 21468289 Free PMC article.
Cited by
-
Guidelines for the Use and Reporting of Patient-Reported Outcomes in Multiple Myeloma Clinical Trials.Cancers (Basel). 2023 Dec 8;15(24):5764. doi: 10.3390/cancers15245764. Cancers (Basel). 2023. PMID: 38136310 Free PMC article.
-
Lived experiences of patients with advanced pancreatic cancer on patient-reported outcomes (PROs) management: a qualitative phenomenological study in Southwest China.BMJ Open. 2025 Jan 28;15(1):e084259. doi: 10.1136/bmjopen-2024-084259. BMJ Open. 2025. PMID: 39880447 Free PMC article.
-
Multiple myeloma.Nat Rev Dis Primers. 2024 Jun 27;10(1):45. doi: 10.1038/s41572-024-00529-7. Nat Rev Dis Primers. 2024. PMID: 38937492 Review.
-
Prognostic impact of patient-reported symptoms in multiple myeloma.Blood Adv. 2025 Feb 25;9(4):884-892. doi: 10.1182/bloodadvances.2024014232. Blood Adv. 2025. PMID: 39637309 Free PMC article.
-
Predictive and Prognostic Significance of Patient-Reported Outcomes for Survival and Adverse Events in Daratumumab-Treated Multiple Myeloma.Eur J Haematol. 2025 Jun;114(6):1020-1031. doi: 10.1111/ejh.14410. Epub 2025 Mar 14. Eur J Haematol. 2025. PMID: 40084510 Free PMC article.
References
-
- Dimopoulos M.A., Moreau P., Terpos E., Mateos M.V., Zweegman S., Cook G., Delforge M., Hájek R., Schjesvold F., Cavo M., et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up(†) Ann. Oncol. 2021;32:309–322. doi: 10.1016/j.annonc.2020.11.014. Erratum in Ann. Oncol. 2021, 5, e528. - DOI - PubMed
-
- Van Valckenborgh E., Schouppe E., Movahedi K., De Bruyne E., Menu E., De Baetselier P., Vanderkerken K., Van Ginderachter J.A. Multiple myeloma induces the immunosuppressive capacity of distinct myeloid-derived suppressor cell subpopulations in the bone marrow. Leukemia. 2012;26:2424–2428. doi: 10.1038/leu.2012.113. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous