Clinical Potential of Circulating Cell-Free DNA (cfDNA) for Longitudinally Monitoring Clinical Outcomes in the First-Line Setting of Non-Small-Cell Lung Cancer (NSCLC): A Real-World Prospective Study
- PMID: 36497493
- PMCID: PMC9735435
- DOI: 10.3390/cancers14236013
Clinical Potential of Circulating Cell-Free DNA (cfDNA) for Longitudinally Monitoring Clinical Outcomes in the First-Line Setting of Non-Small-Cell Lung Cancer (NSCLC): A Real-World Prospective Study
Abstract
Background: Despite the increasing implementation of targeted and immunotherapy-based treatments, the prognosis of patients with advanced NSCLC remains dismal. We prospectively evaluated longitudinal plasma cfDNA kinetics as an early marker of therapeutic efficacy in patients with advanced NSCLC undergoing standard first-line treatments.
Methods: From February 2020 to May 2022, treatment-naïve patients with advanced NSCLC were consecutively enrolled at the Medical Oncology Unit of the Paolo Giaccone University Hospital, Palermo (Italy). We quantified cfDNA in terms of ng/μL using a QubitTM dsDNA HS Assay Kit. The agreement between the cfDNA and radiologic response was evaluated from baseline (T0) to the radiologic evaluation (T1).
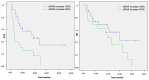
Results: A total of 315 liquid biopsy samples were collected from 63 patients at baseline, with a total of 235 paired plasma samples from 47 patients at disease re-evaluation. A fair concordance was observed between early and durable radiographic and cfDNA response (Cohen's kappa coefficient = 0.001); 11 and 18 patients receiving TKI (Pearson's chi-squared test = 4.278; Cohen's kappa coefficient = 0.039) and IO treatments (Pearson's chi-squared test = 7.481; Cohen's kappa coefficient = 0.006) showed a significant and durable association between cfDNA dynamics and the first radiologic evaluation, whereas among the 18 patients undergoing CT, no significant correlation was observed (Pearson's chi-squared test = 0.720; Cohen's kappa coefficient = 0.396). The ECOG-PS 2 patients presented with the mean baseline cfDNA levels 2.6-fold higher than those with ECOG-PS 0-1 (1.71 vs. 0.65 ng/µL; p = 0.105).
Conclusions: Our real-world study demonstrates that quantitative changes in cfDNA values correlated with responses to therapy and relapse of disease in treatment-naïve patients with advanced NSCLC undergoing TKI- and IO-based treatments.
Keywords: ECOG-PS 2; NSCLC; cfDNA; liquid biopsy; treatment monitoring.
Conflict of interest statement
A.R. reported personal fees from Bristol, Pfizer, Bayer, Kyowa Kirin, Ambrosetti for advisory board activity and a speaker honorarium from Roche Diagnostics, outside from the submitted work. The remaining authors declare no potential conflict of interest.
Figures


References
-
- Russo A., Incorvaia L., Del Re M., Malapelle U., Capoluongo E., Gristina V., Castiglia M., Danesi R., Fassan M., Giuffrè G., et al. The molecular profiling of solid tumors by liquid biopsy: A position paper of the AIOM–SIAPEC-IAP–SIBioC–SIC–SIF Italian Scientific Societies. ESMO Open. 2021;6:100164. doi: 10.1016/j.esmoop.2021.100164. - DOI - PMC - PubMed
-
- Rolfo C., Mack P., Scagliotti G.V., Aggarwal C., Arcila M.E., Barlesi F., Bivona T., Diehn M., Dive C., Dziadziuszko R., et al. Liquid Biopsy for Advanced NSCLC: A Consensus Statement From the International Association for the Study of Lung Cancer. J. Thorac. Oncol. 2021;16:1647–1662. doi: 10.1016/j.jtho.2021.06.017. - DOI - PubMed
-
- Fernandes M.G.O., Sousa C., Reis J.P., Cruz-Martins N., Moura C.S., Guimarães S., Justino A., Pina M.J., Magalhães A., Queiroga H., et al. Liquid biopsy for disease monitoring in non-small cell lung cancer: The link between biology and the clinic. Cells. 2021;10:1912. doi: 10.3390/cells10081912. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

