Oncolytic Viruses for the Treatment of Bladder Cancer: Advances, Challenges, and Prospects
- PMID: 36498574
- PMCID: PMC9738443
- DOI: 10.3390/jcm11236997
Oncolytic Viruses for the Treatment of Bladder Cancer: Advances, Challenges, and Prospects
Abstract
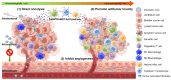
Bladder cancer is one of the most prevalent cancers. Despite recent advancements in bladder cancer therapy, new strategies are still required for improving patient outcomes, particularly for those who experienced Bacille Calmette-Guerin failure and those with locally advanced or metastatic bladder cancer. Oncolytic viruses are either naturally occurring or purposefully engineered viruses that have the ability to selectively infect and lyse tumor cells while avoiding harming healthy cells. In light of this, oncolytic viruses serve as a novel and promising immunotherapeutic strategy for bladder cancer. A wide diversity of viruses, including adenoviruses, herpes simplex virus, coxsackievirus, Newcastle disease virus, vesicular stomatitis virus, alphavirus, and vaccinia virus, have been studied in many preclinical and clinical studies for their potential as oncolytic agents for bladder cancer. This review aims to provide an overview of the advances in oncolytic viruses for the treatment of bladder cancer and highlights the challenges and research directions for the future.
Keywords: bladder cancer; immunotherapy; oncolytic viral therapy; oncolytic virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Oncolytic Immunotherapy for Bladder Cancer Using Coxsackie A21 Virus: Using a Bladder Tumor Precision-Cut Slice Model System to Assess Viral Efficacy.Methods Mol Biol. 2020;2058:249-259. doi: 10.1007/978-1-4939-9794-7_16. Methods Mol Biol. 2020. PMID: 31486043
-
Current status of clinical trials assessing oncolytic virus therapy for urological cancers.Int J Urol. 2017 May;24(5):342-351. doi: 10.1111/iju.13325. Epub 2017 Mar 21. Int J Urol. 2017. PMID: 28326624 Review.
-
Oncolytic Viruses as a Novel Therapeutic Approach for Colorectal Cancer: Mechanisms, Current Advances, and Future Directions.Cancers (Basel). 2025 May 31;17(11):1854. doi: 10.3390/cancers17111854. Cancers (Basel). 2025. PMID: 40507337 Free PMC article. Review.
-
Oncolytic virus therapy: A new era of cancer treatment at dawn.Cancer Sci. 2016 Oct;107(10):1373-1379. doi: 10.1111/cas.13027. Epub 2016 Sep 9. Cancer Sci. 2016. PMID: 27486853 Free PMC article. Review.
-
New frontiers in oncolytic viruses: optimizing and selecting for virus strains with improved efficacy.Biologics. 2018 Feb 9;12:43-60. doi: 10.2147/BTT.S140114. eCollection 2018. Biologics. 2018. PMID: 29445265 Free PMC article. Review.
Cited by
-
IRE1α modulates M1 oncolytic virus sensitivity via ER stress regulation in bladder cancer.Cancer Drug Resist. 2025 Aug 13;8:41. doi: 10.20517/cdr.2025.119. eCollection 2025. Cancer Drug Resist. 2025. PMID: 40843354 Free PMC article.
-
Cell-based and cell-free immunotherapies for glioblastoma: current status and future directions.Front Immunol. 2023 May 25;14:1175118. doi: 10.3389/fimmu.2023.1175118. eCollection 2023. Front Immunol. 2023. PMID: 37304305 Free PMC article. Review.
-
An overview of immune checkpoint inhibitor toxicities in bladder cancer.Toxicol Rep. 2024 Sep 11;13:101732. doi: 10.1016/j.toxrep.2024.101732. eCollection 2024 Dec. Toxicol Rep. 2024. PMID: 39318722 Free PMC article. Review.
References
-
- Safiri S., Kolahi A.A., Naghavi M., Global Burden of Disease Bladder Cancer C. Global, regional and national burden of bladder cancer and its attributable risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the global burden of disease study 2019. BMJ Glob. Health. 2021;6:e004128. doi: 10.1136/bmjgh-2020-004128. - DOI - PMC - PubMed
-
- Babjuk M., Burger M., Capoun O., Cohen D., Comperat E.M., Dominguez Escrig J.L., Gontero P., Liedberg F., Masson-Lecomte A., Mostafid A.H., et al. European association of urology guidelines on non-muscle-invasive bladder cancer (ta, t1, and carcinoma in situ) Eur. Urol. 2022;81:75–94. doi: 10.1016/j.eururo.2021.08.010. - DOI - PubMed
-
- Cathomas R., Lorch A., Bruins H.M., Comperat E.M., Cowan N.C., Efstathiou J.A., Fietkau R., Gakis G., Hernandez V., Espinos E.L., et al. The 2021 updated european association of urology guidelines on metastatic urothelial carcinoma. Eur. Urol. 2022;81:95–103. doi: 10.1016/j.eururo.2021.09.026. - DOI - PubMed
-
- Powles T., Bellmunt J., Comperat E., De Santis M., Huddart R., Loriot Y., Necchi A., Valderrama B.P., Ravaud A., Shariat S.F., et al. Bladder cancer: Esmo clinical practice guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022;33:244–258. doi: 10.1016/j.annonc.2021.11.012. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

