Genetic and Small-Molecule Modulation of Stat3 in a Mouse Model of Crohn's Disease
- PMID: 36498596
- PMCID: PMC9736649
- DOI: 10.3390/jcm11237020
Genetic and Small-Molecule Modulation of Stat3 in a Mouse Model of Crohn's Disease
Abstract
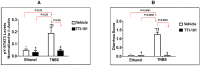
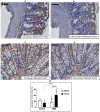
Crohn's disease (CD), is an inflammatory bowel disease that can affect any part of the gastro-intestinal tract (GI) and is associated with an increased risk of gastro-intestinal cancer. In the current study, we determined the role of genetic and small-molecule modulation of STAT3 in a mouse model of CD. STAT3 has 2 isoforms (α, β) which are expressed in most cells in a 4:1 ratio (α: β). STAT3α has pro-inflammatory and anti-apoptotic functions, while STAT3β has contrasting roles. We used an animal model of CD consisting of intrarectal administration of 2,4,6-trinitrobenzene sulfonic acid and examined the severity of CD in transgenic-mice that express only STAT3α (∆β/∆β), as well as in wild-type (WT) mice administered TTI-101 (formerly C188-9), a small molecule STAT3 inhibitor. We determined that clinical manifestations of CD, such as mortality, rectal-bleeding, colonic bleeding, diarrhea, and colon shortening, were exacerbated in ∆β/∆β transgenic versus cage-control WT mice, while they were markedly decreased by TTI-101 treatment of WT mice. TTI-101 treatment also increased apoptosis of pathogenic CD4+ T cells and reduced colon levels of IL-17-positive cells. Our results indicate that STAT3 contributes to CD and that targeting of STAT3 with TTI-101 may be a useful approach to treating CD.
Keywords: Crohn’s disease; STAT3; inflammatory bowel disease.
Conflict of interest statement
D.T. has the following conflict of interest: Baylor College of Medicine, with D.T. as primary inventor, filed patents concerning TTI-101. Tvardi Therapeutics, Inc. (TTI) currently holds an exclusive license to these patents. D.T. owns TTI stock and serves on the TTI Scientific Advisory Board. He has been in compliance with all conflict of interests (COI) policies at Baylor College of Medicine and currently is in compliance with all COI policies at the University of Texas M.D. Anderson Cancer Center, where he relocated in December 2014. The University of Texas MD Anderson Cancer Center has an institutional financial conflict of interest with Tvardi Therapeutics, Inc. related to the research being reported in this manuscript. David Tweardy, Division Head of Internal Medicine, also has a financial interest with Tvardi Therapeutics. Because MD Anderson is committed to the protection of human subjects and the effective management of its financial conflicts of interest in relation to its research activities, MD Anderson has implemented an Institutional Conflict of Interest Management and Monitoring Plan (Plan) to manage and monitor the conflict of interest with respect to MD Anderson’s conduct of this research. The remaining authors have no conflict to disclose.
Figures





References
-
- Juckett G., Trivedi R. Evaluation of chronic diarrhea. Am. Fam. Physician. 2011;84:1119–1126. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

