Zfra Inhibits the TRAPPC6AΔ-Initiated Pathway of Neurodegeneration
- PMID: 36498839
- PMCID: PMC9739312
- DOI: 10.3390/ijms232314510
Zfra Inhibits the TRAPPC6AΔ-Initiated Pathway of Neurodegeneration
Abstract
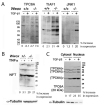
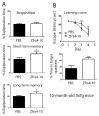
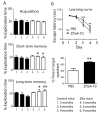
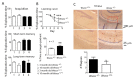
When WWOX is downregulated in middle age, aggregation of a protein cascade, including TRAPPC6AΔ (TPC6AΔ), TIAF1, and SH3GLB2, may start to occur, and the event lasts more than 30 years, which results in amyloid precursor protein (APP) degradation, amyloid beta (Aβ) generation, and neurodegeneration, as shown in Alzheimer's disease (AD). Here, by treating neuroblastoma SK-N-SH cells with neurotoxin MPP+, upregulation and aggregation of TPC6AΔ, along with aggregation of TIAF1, SH3GLB2, Aβ, and tau, occurred. MPP+ is an inducer of Parkinson's disease (PD), suggesting that TPC6AΔ is a common initiator for AD and PD pathogenesis. Zfra, a 31-amino-acid zinc finger-like WWOX-binding protein, is known to restore memory deficits in 9-month-old triple-transgenic (3xTg) mice by blocking the aggregation of TPC6AΔ, SH3GLB2, tau, and amyloid β, as well as inflammatory NF-κB activation. The Zfra4-10 peptide exerted a strong potency in preventing memory loss during the aging of 3-month-old 3xTg mice up to 9 months, as determined by a novel object recognition task (ORT) and Morris water maize analysis. Compared to age-matched wild type mice, 11-month-old Wwox heterozygous mice exhibited memory loss, and this correlates with pT12-WWOX aggregation in the cortex. Together, aggregation of pT12-WWOX may link to TPC6AΔ aggregation for AD progression, with TPC6AΔ aggregation being a common initiator for AD and PD progression.
Keywords: Alzheimer’s disease; SH3GLB2; TIAF1; TRAPPC6A; TRAPPC6AΔ; WWOX; p53; tumor suppressor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Zfra Overrides WWOX in Suppressing the Progression of Neurodegeneration.Int J Mol Sci. 2024 Mar 20;25(6):3507. doi: 10.3390/ijms25063507. Int J Mol Sci. 2024. PMID: 38542478 Free PMC article. Review.
-
Zfra restores memory deficits in Alzheimer's disease triple-transgenic mice by blocking aggregation of TRAPPC6AΔ, SH3GLB2, tau, and amyloid β, and inflammatory NF-κB activation.Alzheimers Dement (N Y). 2017 Mar 6;3(2):189-204. doi: 10.1016/j.trci.2017.02.001. eCollection 2017 Jun. Alzheimers Dement (N Y). 2017. PMID: 29067327 Free PMC article.
-
WWOX dysfunction induces sequential aggregation of TRAPPC6AΔ, TIAF1, tau and amyloid β, and causes apoptosis.Cell Death Discov. 2015 Aug 3;1:15003. doi: 10.1038/cddiscovery.2015.3. eCollection 2015. Cell Death Discov. 2015. PMID: 27551439 Free PMC article.
-
WWOX Phosphorylation, Signaling, and Role in Neurodegeneration.Front Neurosci. 2018 Aug 15;12:563. doi: 10.3389/fnins.2018.00563. eCollection 2018. Front Neurosci. 2018. PMID: 30158849 Free PMC article. Review.
-
Trafficking protein particle complex 6A delta (TRAPPC6AΔ) is an extracellular plaque-forming protein in the brain.Oncotarget. 2015 Feb 28;6(6):3578-89. doi: 10.18632/oncotarget.2876. Oncotarget. 2015. PMID: 25650666 Free PMC article.
Cited by
-
Zfra Overrides WWOX in Suppressing the Progression of Neurodegeneration.Int J Mol Sci. 2024 Mar 20;25(6):3507. doi: 10.3390/ijms25063507. Int J Mol Sci. 2024. PMID: 38542478 Free PMC article. Review.
-
Editorial: The role of STAT3 signaling pathway in tumor progression.Front Oncol. 2023 Feb 17;13:1151862. doi: 10.3389/fonc.2023.1151862. eCollection 2023. Front Oncol. 2023. PMID: 36874126 Free PMC article. No abstract available.
References
-
- Oliveira-Cruz L., Cabrera D. Transforming growth factor beta type I role in neurodegeneration: Implications for Alzheimers disease. Curr. Protein Pept. Sci. 2018;19:1180–1188. - PubMed
-
- Lee M.H., Shih Y.H., Lin S.R., Chang J.Y., Lin Y.H., Sze C.I., Kuo Y.M., Chang N.S. Zfra Restores Memory Deficits in Alzheimer’s Disease Triple-Transgenic Mice by Blocking Aggregation of TRAPPC6A∆, SH3GLB2, Tau, and Amyloid β, and Inflammatory NF-κB Activation. Alzheimers Dement. 2017;3:189–204. doi: 10.1016/j.trci.2017.02.001. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- MOST 108-2320-B-006-020/Ministry of Science and Technology, Taiwan
- MOST 109-2320-B-006-058/Ministry of Science and Technology, Taiwan
- MOST 110-2320-B-006-056/Ministry of Science and Technology, Taiwan
- NHRI-EX107-10734NI/National Health Research Institute, Taiwan
- W81XWH-08-1-0682/United States Department of Defense
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

