Phage-Based Control of Methicillin Resistant Staphylococcus aureus in a Galleria mellonella Model of Implant-Associated Infection
- PMID: 36498843
- PMCID: PMC9740198
- DOI: 10.3390/ijms232314514
Phage-Based Control of Methicillin Resistant Staphylococcus aureus in a Galleria mellonella Model of Implant-Associated Infection
Abstract
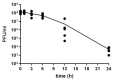
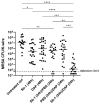
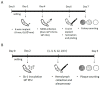
Staphylococcus aureus implant-associated infections are difficult to treat because of the ability of bacteria to form biofilm on medical devices. Here, the efficacy of Sb-1 to control or prevent S. aureus colonization on medical foreign bodies was investigated in a Galleria mellonella larval infection model. For colonization control assays, sterile K-wires were implanted into larva prolegs. After 2 days, larvae were infected with methicillin-resistant S. aureus ATCC 43300 and incubated at 37 °C for a further 2 days, when treatments with either daptomycin (4 mg/kg), Sb-1 (107 PFUs) or a combination of them (3 x/day) were started. For biofilm prevention assays, larvae were pre-treated with either vancomycin (10 mg/kg) or Sb-1 (107 PFUs) before the S. aureus infection. In both experimental settings, K-wires were explanted for colony counting two days after treatment. In comparison to the untreated control, more than a 4 log10 CFU and 1 log10 CFU reduction was observed on K-wires recovered from larvae treated with the Sb-1/daptomycin combination and with their singular administration, respectively. Moreover, pre-infection treatment with Sb-1 was found to prevent K-wire colonization, similarly to vancomycin. Taken together, the obtained results demonstrated the strong potential of the Sb-1 antibiotic combinatory administration or the Sb-1 pretreatment to control or prevent S. aureus-associated implant infections.
Keywords: Galleria mellonella; K-wire; Staphylococcus aureus; antibiotic resistance; biofilm; phage therapy; prosthetic infections; staphylococcal bacteriophages.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Osmon D.R., Berbari E.F., Berendt A.R., Lew D., Zimmerli W., Steckelberg J.M., Rao N., Hanssen A., Wilson W.R. Diagnosis and Management of Prosthetic Joint Infection: Clinical Practice Guidelines by the Infectious Diseases Society of Americaa. Clin. Infect. Dis. 2013;56:e1–e25. doi: 10.1093/cid/cis803. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

