Therapeutic Antibodies in Cancer Treatment in the UK
- PMID: 36498915
- PMCID: PMC9739895
- DOI: 10.3390/ijms232314589
Therapeutic Antibodies in Cancer Treatment in the UK
Abstract
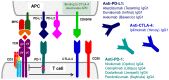
The growing understanding of the molecular mechanisms of carcinogenesis accelerated the development of monoclonal therapeutic antibodies to specifically target multiple cancer pathways. Recombinant protein therapeutics now constitute a large proportion of yearly approved medicines. Oncology, autoimmune diseases and to a smaller degree the prophylaxis of organ transplant rejection are their main application areas. As of the date of this review, 37 monoclonal antibody products are approved for use in cancer treatments in the United Kingdom. Currently, the antibody therapeutics market is dominated by monoclonal immunoglobulins (IgGs). New types of recombinant antibody therapeutics developed more recently include bispecific recombinant antibodies and other recombinantly produced functional proteins. This review focuses on the approved therapeutic antibodies used in cancer treatment in the UK today and describes their antigen targets and molecular mechanisms involved. We provide convenient links to the relevant databases and other relevant resources for all antigens and antibodies mentioned. This review provides a comprehensive summary of the different monoclonal antibodies that are currently in clinical use primarily in malignancy, including their function, which is of importance to those in the medical field and allied specialties.
Keywords: antibody drug conjugates; bispecific T-cell-engager; bispecific antibodies; cancer; cancer treatment; monoclonal therapeutic antibody.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

