Distant Recurrence of a Cerebral Cavernous Malformation in the Vicinity of a Developmental Venous Anomaly: Case Report of Local Oxy-Inflammatory Events
- PMID: 36498972
- PMCID: PMC9736411
- DOI: 10.3390/ijms232314643
Distant Recurrence of a Cerebral Cavernous Malformation in the Vicinity of a Developmental Venous Anomaly: Case Report of Local Oxy-Inflammatory Events
Abstract
Background: Cerebral cavernous malformations (CCMs) are a major type of cerebrovascular lesions of proven genetic origin that occur in either sporadic (sCCM) or familial (fCCM) forms, the latter being inherited as an autosomal dominant condition linked to loss-of-function mutations in three known CCM genes. In contrast to fCCMs, sCCMs are rarely linked to mutations in CCM genes and are instead commonly and peculiarly associated with developmental venous anomalies (DVAs), suggesting distinct origins and common pathogenic mechanisms.
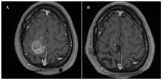
Case report: A hemorrhagic sCCM in the right frontal lobe of the brain was surgically excised from a symptomatic 3 year old patient, preserving intact and pervious the associated DVA. MRI follow-up examination performed periodically up to 15 years after neurosurgery intervention demonstrated complete removal of the CCM lesion and no residual or relapse signs. However, 18 years after surgery, the patient experienced acute episodes of paresthesia due to a distant recurrence of a new hemorrhagic CCM lesion located within the same area as the previous one. A new surgical intervention was, therefore, necessary, which was again limited to the CCM without affecting the pre-existing DVA. Subsequent follow-up examination by contrast-enhanced MRI evidenced a persistent pattern of signal-intensity abnormalities in the bed of the DVA, including hyperintense gliotic areas, suggesting chronic inflammatory conditions.
Conclusions: This case report highlights the possibility of long-term distant recurrence of hemorrhagic sCCMs associated with a DVA, suggesting that such recurrence is secondary to focal sterile inflammatory conditions generated by the DVA.
Keywords: cerebral cavernous malformation (CCM); cerebrovascular diseases; developmental venous anomaly (DVA); oxidative stress; sterile inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

