Immunoproteasome Inhibition Ameliorates Aged Dystrophic Mouse Muscle Environment
- PMID: 36498987
- PMCID: PMC9739773
- DOI: 10.3390/ijms232314657
Immunoproteasome Inhibition Ameliorates Aged Dystrophic Mouse Muscle Environment
Abstract
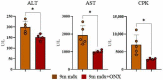
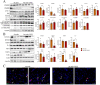
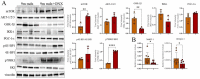
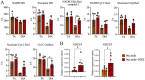
Muscle wasting is a major pathological feature observed in Duchenne muscular dystrophy (DMD) and is the result of the concerted effects of inflammation, oxidative stress and cell senescence. The inducible form of proteasome, or immunoproteasome (IP), is involved in all the above mentioned processes, regulating antigen presentation, cytokine production and immune cell response. IP inhibition has been previously shown to dampen the altered molecular, histological and functional features of 3-month-old mdx mice, the animal model for DMD. In this study, we described the role of ONX-0914, a selective inhibitor of the PSMB8 subunit of immunoproteasome, in ameliorating the pathological traits that could promote muscle wasting progression in older, 9-month-old mdx mice. ONX-0914 reduces the number of macrophages and effector memory T cells in muscle and spleen, while increasing the number of regulatory T cells. It modulates inflammatory markers both in skeletal and cardiac muscle, possibly counteracting heart remodeling and hypertrophy. Moreover, it buffers oxidative stress by improving mitochondrial efficiency. These changes ultimately lead to a marked decrease of fibrosis and, potentially, to more controlled myofiber degeneration/regeneration cycles. Therefore, ONX-0914 is a promising molecule that may slow down muscle mass loss, with relatively low side effects, in dystrophic patients with moderate to advanced disease.
Keywords: aging; immunoproteasome; inflammation; muscle mass; sarcopenia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Ubaida-Mohien C., Lyashkov A., Gonzalez-Freire M., Tharakan R., Shardell M., Moaddel R., Semba R.D., Chia C.W., Gorospe M., Sen R., et al. Discovery proteomics in aging human skeletal muscle finds change in spliceosome, immunity, proteostasis and mitochondria. eLife. 2019;8:e49874. doi: 10.7554/eLife.49874. - DOI - PMC - PubMed
-
- Brehm M.A., Kempen J.C., van der Kooi A.J., de Groot I.J., van den Bergen J.C., Verschuuren J.J., Niks E.H., Harlaar J. Age-related longitudinal changes in metabolic energy expenditure during walking in boys with Duchenne muscular dystrophy. PLoS ONE. 2014;9:e115200. doi: 10.1371/journal.pone.0115200. - DOI - PMC - PubMed

