Red Algae “Sarcodia suieae” Acetyl-Xylogalactan Downregulate Heat-Induced Macrophage Stress Factors Ddit3 and Hyou1 Compared to the Aquatic Animal Model of Nile Tilapia (Oreochromis niloticus) Brain Arachidonic Acid Expression
- PMID: 36498988
- PMCID: PMC9737935
- DOI: 10.3390/ijms232314662
Red Algae “Sarcodia suieae” Acetyl-Xylogalactan Downregulate Heat-Induced Macrophage Stress Factors Ddit3 and Hyou1 Compared to the Aquatic Animal Model of Nile Tilapia (Oreochromis niloticus) Brain Arachidonic Acid Expression
Abstract
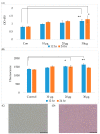
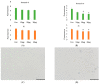
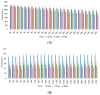
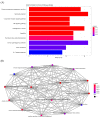
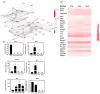
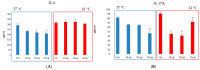
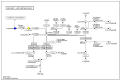
Anthropogenic climate change is known to be an increased stress that affects aquatic animal behavior and physiological alternations, which can induce the animal's death. In order to known whether the extracted acetyl-xylogalactan function on the regulation of the external high temperature induced death, we first selected the mammalian cell line "RAW 264.7" used in the previous experiment to evaluate the extracted acetyl-xylogalactan function. We aimed to evaluate the effects of the acetyl-xylogalactan on the RAW 264.7 macrophages and Nile Tilapia stress factor expression under the heat environment. In the in vitro cell observation, we assessed the cell survival, phagocytic activity, intracellular Ca2+ level, mitochondria potential exchange, apoptotic assay findings, galactosidase activity, RNA-seq by NGS and real-time polymerase chain reaction (QPCR) expression. In the in vivo Nile Tilapia observation aimed to evaluate the blood biochemical indicator, brain metabolites exchange and the liver morphology. In our evaluation of RAW 264.7 macrophages, the RNA sequencing and real-time polymerase chain reaction (PCR) was shown to upregulate the expression of the anti-apoptosis Cflar gene and downregulate the expression of the apoptosis factors Ddit3 and Hyou1 to protect macrophages under heat stress. We already knew the extracted acetyl-xylogalactan function on the mammalian "RAW 264.7" system. Following, we used the aquatic Nile Tilapia model as the anthropogenic climate change high temperature experiment. After feeding the Nile Tilapia with the acetyl-xylogalactan, it was found to reduce the brain arachidonic acid (AA) production, which is related to the NF-κB-induced apoptosis mechanism. Combined with the in vitro and in vivo findings, the acetyl-xylogalactan was able to reduce the heat induced cell or tissue stress.
Keywords: Sarcodia suieae; acetyl-xylogalactan; apoptosis; environment; stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Portner H.O., Langenbuch M., Michaelidis B. Synergistic effects of temperature extremes, hypoxia, and increases in CO2 on marine animals: From Earth history to global change. J. Geophys. Res. Oceans. 2005;110 doi: 10.1029/2004JC002561. - DOI
-
- Firmino M., Weis S.N., Souza J.M., Gomes B.R., Mól A.R., Mortari M.R., Souza G.E.P., Coca G.C., Williams T.C.R., Fontes W., et al. Label-free quantitative proteomics of rat hypothalamus under fever induced by LPS and PGE2. J. Proteom. 2018;187:182–199. doi: 10.1016/j.jprot.2018.07.018. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

