Modulation of the Inflammatory Response in Polycystic Ovary Syndrome (PCOS)-Searching for Epigenetic Factors
- PMID: 36498989
- PMCID: PMC9736994
- DOI: 10.3390/ijms232314663
Modulation of the Inflammatory Response in Polycystic Ovary Syndrome (PCOS)-Searching for Epigenetic Factors
Abstract
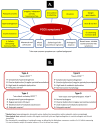
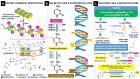
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women of reproductive age. Despite its incidence, the syndrome is poorly understood and remains underdiagnosed, and female patients are diagnosed with a delay. The heterogenous nature of this complex disorder results from the combined occurrence of genetic, environmental, endocrine, and behavioral factors. Primary clinical manifestations of PCOS are derived from the excess of androgens (anovulation, polycystic ovary morphology, lack of or scanty, irregular menstrual periods, acne and hirsutism), whereas the secondary manifestations include multiple metabolic, cardiovascular, and psychological disorders. Dietary and lifestyle factors play important roles in the development and course of PCOS, which suggests strong epigenetic and environmental influences. Many studies have shown a strong association between PCOS and chronic, low-grade inflammation both in the ovarian tissue and throughout the body. In the vast majority of PCOS patients, elevated values of inflammatory markers or their gene markers have been reported. Development of the vicious cycle of the chronic inflammatory state in PCOS is additionally stimulated by hyperinsulinemia and obesity. Changes in DNA methylation, histone acetylation and noncoding RNA levels are presented in this review in the context of oxidative stress, reactive oxygen species, and inflammatory signaling in PCOS. Epigenetic modulation of androgenic activity in response to inflammatory signaling is also discussed.
Keywords: androgenic activity modulation; epigenetic factors; inflammatory response; insulin resistance; mitochondrial dysfunction; oxidative stress; polycystic ovary syndrome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

