Optimal Intravenous Administration Procedure for Efficient Delivery of Canine Adipose-Derived Mesenchymal Stem Cells
- PMID: 36499004
- PMCID: PMC9740176
- DOI: 10.3390/ijms232314681
Optimal Intravenous Administration Procedure for Efficient Delivery of Canine Adipose-Derived Mesenchymal Stem Cells
Abstract
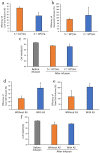
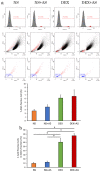
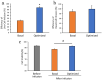
Mesenchymal stem cells (MSC) are currently being investigated for their therapeutic applications in a wide range of diseases. Although many studies examined peripheral venous administration of MSC, few have investigated the detailed intravenous administration procedures of MSC from their preparation until they enter the body. The current study therefore aimed to explore the most efficient infusion procedure for MSC delivery by preparing and infusing them under various conditions. Canine adipose-derived mesenchymal stem cells (cADSC) were infused using different infusion apparatuses, suspension solutions, allogenic serum supplementation, infusion time and rates, and cell densities, respectively. Live and dead cell counts were then assessed by manual measurements and flow cytometry. Efficiency of live- and dead-cell infusion and cell viability were calculated from the measured cell counts and compared under each condition. Efficiency of live-cell infusion differed significantly according to the infusion apparatus, infusion rate, and combination of cell density and serum supplementation. Cell viability after infusion differed significantly between the infusion apparatuses. The optimal infusion procedure resulting in the highest cell delivery and viability involved suspending cADSC in normal saline supplemented with 5% allogenic serum at a density of 5 × 105 cells/mL, and infusing them using an automatic infusion device for 15 min. This procedure is therefore recommended as the standard procedure for the intravenous administration of ADSC in terms of cell-delivery efficiency.
Keywords: adipose-derived mesenchymal stem cells; canine; cell delivery; cell viability; intravenous administration.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures


Similar articles
-
Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients With Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]).Circ Res. 2017 Oct 27;121(10):1192-1204. doi: 10.1161/CIRCRESAHA.117.310712. Epub 2017 Sep 26. Circ Res. 2017. PMID: 28974553 Free PMC article. Clinical Trial.
-
Intravenous injection of allogenic canine mesenchymal stem cells in 40 client-owned dogs: a safety assessment in veterinary clinical trials.BMC Vet Res. 2024 Aug 22;20(1):375. doi: 10.1186/s12917-024-04216-3. BMC Vet Res. 2024. PMID: 39174969 Free PMC article.
-
Mesenchymal Stromal Cells Are Retained in the Porcine Renal Cortex Independently of Their Metabolic State After Renal Intra-Arterial Infusion.Stem Cells Dev. 2019 Sep 15;28(18):1224-1235. doi: 10.1089/scd.2019.0105. Epub 2019 Aug 1. Stem Cells Dev. 2019. PMID: 31280676
-
Systemic and local delivery of mesenchymal stem cells for heart renovation: Challenges and innovations.Eur J Pharmacol. 2020 Jun 5;876:173049. doi: 10.1016/j.ejphar.2020.173049. Epub 2020 Mar 3. Eur J Pharmacol. 2020. PMID: 32142771 Review.
-
Neuroprotection by mesenchymal stem cell (MSC) administration is enhanced by local cooling infusion (LCI) in ischemia.Brain Res. 2019 Dec 1;1724:146406. doi: 10.1016/j.brainres.2019.146406. Epub 2019 Aug 24. Brain Res. 2019. PMID: 31454517 Review.
Cited by
-
Canine adipose-derived mesenchymal stromal cells inhibit the growth of canine hematologic malignancy cell lines.Regen Ther. 2025 Jan 4;28:301-313. doi: 10.1016/j.reth.2024.12.019. eCollection 2025 Mar. Regen Ther. 2025. PMID: 39867136 Free PMC article.
-
Effective enhancement of the immunomodulatory capacity of canine adipose-derived mesenchymal stromal cells on colitis by priming with colon tissue from mice with colitis.Front Vet Sci. 2024 Aug 8;11:1437648. doi: 10.3389/fvets.2024.1437648. eCollection 2024. Front Vet Sci. 2024. PMID: 39176394 Free PMC article.
References
-
- Markov A., Thangavelu L., Aravindhan S., Zekiy A.O., Jarahian M., Chartrand M.S., Pathak Y., Marofi F., Shamlou S., Hassanzadeh A. Mesenchymal stem/stromal cells as a valuable source for the treatment of immune-mediated disorders. Stem. Cell Res. Ther. 2021;12:192. doi: 10.1186/s13287-021-02265-1. - DOI - PMC - PubMed
-
- Dominici M., Blanc K.L., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

