The Protective Role of Apelin in the Early Stages of Diabetic Retinopathy
- PMID: 36499009
- PMCID: PMC9740800
- DOI: 10.3390/ijms232314680
The Protective Role of Apelin in the Early Stages of Diabetic Retinopathy
Abstract
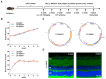
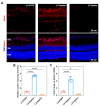
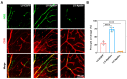
Diabetic retinopathy (DR) is one of the most common and serious microvascular complications of diabetes. Although current treatments can control the progression of DR to a certain extent, there is no effective treatment for early DR. Apart from vascular endothelial growth factor, it has been noted that the apelin/APJ system contributes to the pathogenesis of DR. We used a high-fat diet/streptozotocin-induced type 2 diabetic mouse model. The mice were divided into a lentivirus control group (LV-EGFP), an apelin-overexpression group (LV-Apelin+), and an apelin-knockdown group (LV-Apelin-), all of which were administrated intravitreal injections. LV-Apelin+ ameliorated the loss of pericytes in DR mice, whereas LV-Apelin- aggravated the loss of pericytes. Similarly, LV-Apelin+ reduced the leakage of retinal vessels, whereas LV-Apelin- exacerbated it. The genes and signaling pathway related to cell adhesion molecules were downregulated, whereas the cell-cell tight junctions and anti-apoptotic genes were upregulated in response to apelin overexpression. However, the alterations of these same genes and signaling pathways were reversed in the case of apelin knockdown. Additionally, LV-Apelin+ increased ZO-1 and occludin levels, whereas LV-Apelin- decreased them. Our results suggest that apelin can reduce vascular leakage by protecting pericytes, which offers a promising new direction for the early treatment of DR.
Keywords: apelin; early stages of diabetic retinopathy; pericytes; protective role; vascular leakage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Circular RNA-ZNF532 regulates diabetes-induced retinal pericyte degeneration and vascular dysfunction.J Clin Invest. 2020 Jul 1;130(7):3833-3847. doi: 10.1172/JCI123353. J Clin Invest. 2020. PMID: 32343678 Free PMC article.
-
Low expression of microRNA-15b promotes the proliferation of retinal capillary endothelial cells and pericytes by up-regulating VEGFA in diabetic rats.Eur Rev Med Pharmacol Sci. 2019 Jul;23(14):6018-6025. doi: 10.26355/eurrev_201907_18413. Eur Rev Med Pharmacol Sci. 2019. PMID: 31364104
-
Diabetes-enhanced tumor necrosis factor-alpha production promotes apoptosis and the loss of retinal microvascular cells in type 1 and type 2 models of diabetic retinopathy.Am J Pathol. 2008 May;172(5):1411-8. doi: 10.2353/ajpath.2008.071070. Epub 2008 Apr 10. Am J Pathol. 2008. PMID: 18403591 Free PMC article.
-
Multiple roles of apelin/APJ system in eye diseases.Peptides. 2022 Jun;152:170767. doi: 10.1016/j.peptides.2022.170767. Epub 2022 Feb 15. Peptides. 2022. PMID: 35181348 Review.
-
[Pathological role of apelin in angiogenic eye disease].Yakugaku Zasshi. 2011;131(8):1201-6. doi: 10.1248/yakushi.131.1201. Yakugaku Zasshi. 2011. PMID: 21804324 Review. Japanese.
Cited by
-
The potential of serum elabela levels as a marker of diabetic retinopathy: results from a pilot cross-sectional study.PeerJ. 2025 Jan 8;13:e18841. doi: 10.7717/peerj.18841. eCollection 2025. PeerJ. 2025. PMID: 39802191 Free PMC article.
-
New Markers for the Assessment of Microvascular Complications in Patients with Metabolic Syndrome.Metabolites. 2025 Mar 10;15(3):184. doi: 10.3390/metabo15030184. Metabolites. 2025. PMID: 40137149 Free PMC article. Review.
References
-
- Cui Y., Zhang M., Zhang L., Zhang L., Kuang J., Zhang G., Liu Q., Guo H., Meng Q. Prevalence and risk factors for diabetic retinopathy in a cross-sectional population-based study from rural southern China: Dongguan Eye Study. BMJ Open. 2019;9:e023586. doi: 10.1136/bmjopen-2018-023586. - DOI - PMC - PubMed
-
- Caporarello N., D’Angeli F., Cambria M.T., Candido S., Giallongo C., Salmeri M., Lombardo C., Longo A., Giurdanella G., Anfuso C.D., et al. Pericytes in Microvessels: From “Mural” Function to Brain and Retina Regeneration. Int. J. Mol. Sci. 2019;20:6351. doi: 10.3390/ijms20246351. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 82101142, 82070948/National Natural Science Foundation of China
- 2021M692250/China Postdoctoral Science Foundation
- DFL20220301/Beijing Hospitals Authority's Ascent Plan
- 2020027/Beijing Talent Project
- SYGX202010/Shunyi District Beijing Science and technology achievements transformation coordination and service platform construction fund
LinkOut - more resources
Full Text Sources
Medical

