Comparative Efficiency of Gene-Activated Matrices Based on Chitosan Hydrogel and PRP Impregnated with BMP2 Polyplexes for Bone Regeneration
- PMID: 36499056
- PMCID: PMC9735524
- DOI: 10.3390/ijms232314720
Comparative Efficiency of Gene-Activated Matrices Based on Chitosan Hydrogel and PRP Impregnated with BMP2 Polyplexes for Bone Regeneration
Abstract
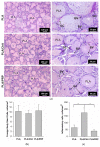
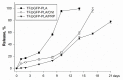
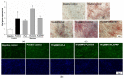
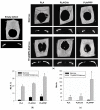
Gene therapy is one of the most promising approaches in regenerative medicine. Gene-activated matrices provide stable gene expression and the production of osteogenic proteins in situ to stimulate osteogenesis and bone repair. In this study, we developed new gene-activated matrices based on polylactide granules (PLA) impregnated with BMP2 polyplexes and included in chitosan hydrogel or PRP-based fibrin hydrogel. The matrices showed high biocompatibility both in vitro with mesenchymal stem cells and in vivo when implanted intramuscularly in rats. The use of porous PLA granules allowed the inclusion of a high concentration of polyplexes, and the introduction of the granules into hydrogel provided the gradual release of the plasmid constructs. All gene-activated matrices showed transfecting ability and ensured long-term gene expression and the production of target proteins in vitro. At the same time, the achieved concentration of BMP-2 was sufficient to induce osteogenic differentiation of MSCs. When implanted into critical-size calvarial defects in rats, all matrices with BMP2 polyplexes led to new bone formation. The most significant effect on osteoinduction was observed for the PLA/PRP matrices. Thus, the developed gene-activated matrices were shown to be safe and effective osteoplastic materials. PLA granules and PRP-based fibrin hydrogel containing BMP2 polyplexes were shown to be the most promising for future applications in bone regeneration.
Keywords: bone regeneration; chitosan; gene-activated matrices; plasmid DNA; platelet rich plasma; polylactide granules.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
Figures






Similar articles
-
Adenovirus-Based Gene Therapy for Bone Regeneration: A Comparative Analysis of In Vivo and Ex Vivo BMP2 Gene Delivery.Cells. 2023 Jul 1;12(13):1762. doi: 10.3390/cells12131762. Cells. 2023. PMID: 37443796 Free PMC article.
-
Influence of the Degree of Deacetylation of Chitosan and BMP-2 Concentration on Biocompatibility and Osteogenic Properties of BMP-2/PLA Granule-Loaded Chitosan/β-Glycerophosphate Hydrogels.Molecules. 2021 Jan 7;26(2):261. doi: 10.3390/molecules26020261. Molecules. 2021. PMID: 33430198 Free PMC article.
-
The combination of nano-calcium sulfate/platelet rich plasma gel scaffold with BMP2 gene-modified mesenchymal stem cells promotes bone regeneration in rat critical-sized calvarial defects.Stem Cell Res Ther. 2017 May 25;8(1):122. doi: 10.1186/s13287-017-0574-6. Stem Cell Res Ther. 2017. PMID: 28545565 Free PMC article.
-
Combination of Controlled Release Platelet-Rich Plasma Alginate Beads and Bone Morphogenetic Protein-2 Genetically Modified Mesenchymal Stem Cells for Bone Regeneration.J Periodontol. 2016 Apr;87(4):470-80. doi: 10.1902/jop.2016.150487. Epub 2016 Jan 8. J Periodontol. 2016. PMID: 26745613 Free PMC article.
-
Matrices Activated with Messenger RNA.J Funct Biomater. 2023 Jan 15;14(1):48. doi: 10.3390/jfb14010048. J Funct Biomater. 2023. PMID: 36662095 Free PMC article. Review.
Cited by
-
Women's contribution to stem cell research for osteoarthritis: an opinion paper.Front Cell Dev Biol. 2023 Dec 19;11:1209047. doi: 10.3389/fcell.2023.1209047. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38174070 Free PMC article. Review. No abstract available.
-
Platelet-rich plasma-contained drug delivery systems to treat orthopedic injuries.Int J Pharm X. 2025 Aug 10;10:100372. doi: 10.1016/j.ijpx.2025.100372. eCollection 2025 Dec. Int J Pharm X. 2025. PMID: 40895344 Free PMC article. Review.
-
Special Issue "Stem Cell Biology & Regenerative Medicine".Int J Mol Sci. 2023 Aug 16;24(16):12855. doi: 10.3390/ijms241612855. Int J Mol Sci. 2023. PMID: 37629035 Free PMC article.
-
Osteogenesis Enhancement with 3D Printed Gene-Activated Sodium Alginate Scaffolds.Gels. 2023 Apr 7;9(4):315. doi: 10.3390/gels9040315. Gels. 2023. PMID: 37102926 Free PMC article.
-
Adenovirus-Based Gene Therapy for Bone Regeneration: A Comparative Analysis of In Vivo and Ex Vivo BMP2 Gene Delivery.Cells. 2023 Jul 1;12(13):1762. doi: 10.3390/cells12131762. Cells. 2023. PMID: 37443796 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

