Pathogenesis of α-Synuclein in Parkinson's Disease: From a Neuron-Glia Crosstalk Perspective
- PMID: 36499080
- PMCID: PMC9739123
- DOI: 10.3390/ijms232314753
Pathogenesis of α-Synuclein in Parkinson's Disease: From a Neuron-Glia Crosstalk Perspective
Abstract
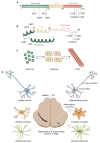
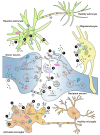
Parkinson's disease (PD) is a progressive neurodegenerative disorder. The classical behavioral defects of PD patients involve motor symptoms such as bradykinesia, tremor, and rigidity, as well as non-motor symptoms such as anosmia, depression, and cognitive impairment. Pathologically, the progressive loss of dopaminergic (DA) neurons in the substantia nigra (SN) and the accumulation of α-synuclein (α-syn)-composed Lewy bodies (LBs) and Lewy neurites (LNs) are key hallmarks. Glia are more than mere bystanders that simply support neurons, they actively contribute to almost every aspect of neuronal development and function; glial dysregulation has been implicated in a series of neurodegenerative diseases including PD. Importantly, amounting evidence has added glial activation and neuroinflammation as new features of PD onset and progression. Thus, gaining a better understanding of glia, especially neuron-glia crosstalk, will not only provide insight into brain physiology events but also advance our knowledge of PD pathologies. This review addresses the current understanding of α-syn pathogenesis in PD, with a focus on neuron-glia crosstalk. Particularly, the transmission of α-syn between neurons and glia, α-syn-induced glial activation, and feedbacks of glial activation on DA neuron degeneration are thoroughly discussed. In addition, α-syn aggregation, iron deposition, and glial activation in regulating DA neuron ferroptosis in PD are covered. Lastly, we summarize the preclinical and clinical therapies, especially targeting glia, in PD treatments.
Keywords: Parkinson’s disease; glia; neuroinflammation; neuron-glia crosstalk; α-synuclein.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

