Understanding In Vitro Pathways to Drug Discovery for TDP-43 Proteinopathies
- PMID: 36499097
- PMCID: PMC9738080
- DOI: 10.3390/ijms232314769
Understanding In Vitro Pathways to Drug Discovery for TDP-43 Proteinopathies
Abstract
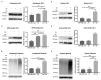
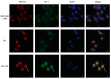
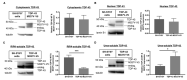
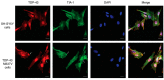
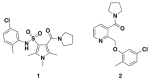
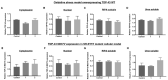
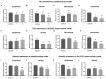
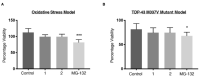
The use of cellular models is a common means to investigate the potency of therapeutics in pre-clinical drug discovery. However, there is currently no consensus on which model most accurately replicates key aspects of amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) pathology, such as accumulation of insoluble, cytoplasmic transactive response DNA-binding protein (TDP-43) and the formation of insoluble stress granules. Given this, we characterised two TDP-43 proteinopathy cellular models that were based on different aetiologies of disease. The first was a sodium arsenite-induced chronic oxidative stress model and the second expressed a disease-relevant TDP-43 mutation (TDP-43 M337V). The sodium arsenite model displayed most aspects of TDP-43, stress granule and ubiquitin pathology seen in human ALS/FTD donor tissue, whereas the mutant cell line only modelled some aspects. When these two cellular models were exposed to small molecule chemical probes, different effects were observed across the two models. For example, a previously disclosed sulfonamide compound decreased cytoplasmic TDP-43 and increased soluble levels of stress granule marker TIA-1 in the cellular stress model without impacting these levels in the mutant cell line. This study highlights the challenges of using cellular models in lead development during drug discovery for ALS and FTD and reinforces the need to perform assessments of novel therapeutics across a variety of cell lines and aetiological models.
Keywords: TDP-43; amyotrophic lateral sclerosis; frontotemporal dementia; proteinopathy; stress granules.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Nuclear transport dysfunction: a common theme in amyotrophic lateral sclerosis and frontotemporal dementia.J Neurochem. 2016 Aug;138 Suppl 1:134-44. doi: 10.1111/jnc.13642. Epub 2016 Jun 15. J Neurochem. 2016. PMID: 27087014 Review.
-
Downregulation of microRNA-9 in iPSC-derived neurons of FTD/ALS patients with TDP-43 mutations.PLoS One. 2013 Oct 15;8(10):e76055. doi: 10.1371/journal.pone.0076055. eCollection 2013. PLoS One. 2013. PMID: 24143176 Free PMC article.
-
Emerging Therapies and Novel Targets for TDP-43 Proteinopathy in ALS/FTD.Neurotherapeutics. 2022 Jul;19(4):1061-1084. doi: 10.1007/s13311-022-01260-5. Epub 2022 Jul 5. Neurotherapeutics. 2022. PMID: 35790708 Free PMC article. Review.
-
Cytoplasmic TDP-43 is involved in cell fate during stress recovery.Hum Mol Genet. 2021 Dec 27;31(2):166-175. doi: 10.1093/hmg/ddab227. Hum Mol Genet. 2021. PMID: 34378050 Free PMC article.
-
TDP-43 Proteinopathy and ALS: Insights into Disease Mechanisms and Therapeutic Targets.Neurotherapeutics. 2015 Apr;12(2):352-63. doi: 10.1007/s13311-015-0338-x. Neurotherapeutics. 2015. PMID: 25652699 Free PMC article. Review.
Cited by
-
Molecular Mechanisms of Dementia.Int J Mol Sci. 2023 Aug 22;24(17):13027. doi: 10.3390/ijms241713027. Int J Mol Sci. 2023. PMID: 37685834 Free PMC article.
References
-
- Mackenzie I.R., Bigio E.H., Ince P.G., Geser F., Neumann M., Cairns N.J., Kwong L.K., Forman M.S., Ravits J., Stewart H. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann. Neurol. 2007;61:427–434. doi: 10.1002/ana.21147. - DOI - PubMed
-
- McGurk L., Gomes E., Guo L., Mojsilovic-Petrovic J., Tran V., Kalb R.G., Shorter J., Bonini N.M. Poly (ADP-ribose) prevents pathological phase separation of TDP-43 by promoting liquid demixing and stress granule localization. Mol. Cell. 2018;71:703–717. doi: 10.1016/j.molcel.2018.07.002. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

