Whole-Head Functional Near-Infrared Spectroscopy as an Ecological Monitoring Tool for Assessing Cortical Activity in Parkinson's Disease Patients at Different Stages
- PMID: 36499223
- PMCID: PMC9736501
- DOI: 10.3390/ijms232314897
Whole-Head Functional Near-Infrared Spectroscopy as an Ecological Monitoring Tool for Assessing Cortical Activity in Parkinson's Disease Patients at Different Stages
Abstract
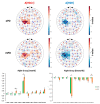
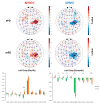
Functional near-infrared spectroscopy (fNIRS) is increasingly employed as an ecological neuroimaging technique in assessing age-related chronic neurological disorders, such as Parkinson's disease (PD), mainly providing a cross-sectional characterization of clinical phenotypes in ecological settings. Current fNIRS studies in PD have investigated the effects of motor and non-motor impairment on cortical activity during gait and postural stability tasks, but no study has employed fNIRS as an ecological neuroimaging tool to assess PD at different stages. Therefore, in this work, we sought to investigate the cortical activity of PD patients during a motor grasping task and its relationship with both the staging of the pathology and its clinical variables. This study considered 39 PD patients (age 69.0 ± 7.64, 38 right-handed), subdivided into two groups at different stages by the Hoehn and Yahr (HY) scale: early PD (ePD; N = 13, HY = [1; 1.5]) and moderate PD (mPD; N = 26, HY = [2; 2.5; 3]). We employed a whole-head fNIRS system with 102 measurement channels to monitor brain activity. Group-level activation maps and region of interest (ROI) analysis were computed for ePD, mPD, and ePD vs. mPD contrasts. A ROI-based correlation analysis was also performed with respect to contrasted subject-level fNIRS data, focusing on age, a Cognitive Reserve Index questionnaire (CRIQ), disease duration, the Unified Parkinson's Disease Rating Scale (UPDRS), and performances in the Stroop Color and Word (SCW) test. We observed group differences in age, disease duration, and the UPDRS, while no significant differences were found for CRIQ or SCW scores. Group-level activation maps revealed that the ePD group presented higher activation in motor and occipital areas than the mPD group, while the inverse trend was found in frontal areas. Significant correlations with CRIQ, disease duration, the UPDRS, and the SCW were mostly found in non-motor areas. The results are in line with current fNIRS and functional and anatomical MRI scientific literature suggesting that non-motor areas-primarily the prefrontal cortex area-provide a compensation mechanism for PD motor impairment. fNIRS may serve as a viable support for the longitudinal assessment of therapeutic and rehabilitation procedures, and define new prodromal, low-cost, and ecological biomarkers of disease progression.
Keywords: Parkinson Disease; brain activation mapping; clinical fNIRS translation; continuous wave functional near-infrared spectroscopy; functional near-infrared signal processing; hemispheric hemodynamic response; motor tasks; rehabilitation monitoring.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

