IL-1RAP, a Key Therapeutic Target in Cancer
- PMID: 36499246
- PMCID: PMC9735758
- DOI: 10.3390/ijms232314918
IL-1RAP, a Key Therapeutic Target in Cancer
Abstract
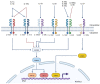
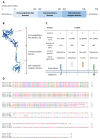
Cancer is a major cause of death worldwide and especially in high- and upper-middle-income countries. Despite recent progress in cancer therapies, such as chimeric antigen receptor T (CAR-T) cells or antibody-drug conjugate (ADC), new targets expressed by the tumor cells need to be identified in order to selectively drive these innovative therapies to tumors. In this context, IL-1RAP recently showed great potential to become one of these new targets for cancer therapy. IL-1RAP is highly involved in the inflammation process through the interleukins 1, 33, and 36 (IL-1, IL-33, IL-36) signaling pathways. Inflammation is now recognized as a hallmark of carcinogenesis, suggesting that IL-1RAP could play a role in cancer development and progression. Furthermore, IL-1RAP was found overexpressed on tumor cells from several hematological and solid cancers, thus confirming its potential involvement in carcinogenesis. This review will first describe the structure and genetics of IL-1RAP as well as its role in tumor development. Finally, a focus will be made on the therapies based on IL-1RAP targeting, which are now under preclinical or clinical development.
Keywords: IL-1R family; IL-1RAP; cancer; innovative therapies; metastasis.
Conflict of interest statement
C.F., Founder and Shareholder of CanCell Therapeutics, 25000 Besançon, France.
Figures




References
-
- Dagenais G.R., Leong D.P., Rangarajan S., Lanas F., Lopez-Jaramillo P., Gupta R., Diaz R., Avezum A., Oliveira G.B.F., Wielgosz A., et al. Variations in common diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): A prospective cohort study. Lancet. 2020;395:785–794. doi: 10.1016/S0140-6736(19)32007-0. - DOI - PubMed
-
- Salas-Benito D., Perez-Gracia J.L., Ponz-Sarvise M., Rodriguez-Ruiz M.E., Martinez-Forero I., Castanon E., Lopez-Picazo J.M., Sanmamed M.F., Melero I. Paradigms on Immunotherapy Combinations with Chemotherapy. Cancer Discov. 2021;11:1353–1367. doi: 10.1158/2159-8290.CD-20-1312. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

