Reduction of Obesity and Insulin Resistance through Dual Targeting of VAT and BAT by a Novel Combination of Metabolic Cofactors
- PMID: 36499250
- PMCID: PMC9738317
- DOI: 10.3390/ijms232314923
Reduction of Obesity and Insulin Resistance through Dual Targeting of VAT and BAT by a Novel Combination of Metabolic Cofactors
Abstract
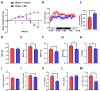
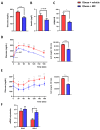
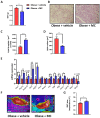
Obesity is an epidemic disease worldwide, characterized by excessive fat accumulation associated with several metabolic perturbations, such as metabolic syndrome, insulin resistance, hypertension, and dyslipidemia. To improve this situation, a specific combination of metabolic cofactors (MC) (betaine, N-acetylcysteine, L-carnitine, and nicotinamide riboside) was assessed as a promising treatment in a high-fat diet (HFD) mouse model. Obese animals were distributed into two groups, orally treated with the vehicle (obese + vehicle) or with the combination of metabolic cofactors (obese + MC) for 4 weeks. Body and adipose depots weights; insulin and glucose tolerance tests; indirect calorimetry; and thermography assays were performed at the end of the intervention. Histological analysis of epidydimal white adipose tissue (EWAT) and brown adipose tissue (BAT) was carried out, and the expression of key genes involved in both fat depots was characterized by qPCR. We demonstrated that MC supplementation conferred a moderate reduction of obesity and adiposity, an improvement in serum glucose and lipid metabolic parameters, an important improvement in lipid oxidation, and a decrease in adipocyte hypertrophy. Moreover, MC-treated animals presented increased adipose gene expression in EWAT related to lipolysis and fatty acid oxidation. Furthermore, MC supplementation reduced glucose intolerance and insulin resistance, with an increased expression of the glucose transporter Glut4; and decreased fat accumulation in BAT, raising non-shivering thermogenesis. This treatment based on a specific combination of metabolic cofactors mitigates important pathophysiological characteristics of obesity, representing a promising clinical approach to this metabolic disease.
Keywords: adipose tissue; insulin resistance; metabolic cofactors; obesity; thermogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Adipocyte-specific Hypoxia-inducible gene 2 promotes fat deposition and diet-induced insulin resistance.Mol Metab. 2016 Sep 28;5(12):1149-1161. doi: 10.1016/j.molmet.2016.09.009. eCollection 2016 Dec. Mol Metab. 2016. PMID: 27900258 Free PMC article.
-
Time-restricted feeding improves metabolic syndrome by activating thermogenesis in brown adipose tissue and reducing inflammatory markers.Front Immunol. 2025 Jan 24;16:1501850. doi: 10.3389/fimmu.2025.1501850. eCollection 2025. Front Immunol. 2025. PMID: 39925816 Free PMC article.
-
Brown adipocyte mineralocorticoid receptor deficiency impairs metabolic regulation in diet-induced obese mice.J Lipid Res. 2023 Nov;64(11):100449. doi: 10.1016/j.jlr.2023.100449. Epub 2023 Sep 20. J Lipid Res. 2023. PMID: 37734559 Free PMC article.
-
A cardiac amino-terminal GRK2 peptide inhibits insulin resistance yet enhances maladaptive cardiovascular and brown adipose tissue remodeling in females during diet-induced obesity.J Mol Cell Cardiol. 2023 Oct;183:81-97. doi: 10.1016/j.yjmcc.2023.09.001. Epub 2023 Sep 14. J Mol Cell Cardiol. 2023. PMID: 37714510 Free PMC article. Review.
-
Emerging Role of AMPK in Brown and Beige Adipose Tissue (BAT): Implications for Obesity, Insulin Resistance, and Type 2 Diabetes.Curr Diab Rep. 2018 Aug 17;18(10):80. doi: 10.1007/s11892-018-1049-6. Curr Diab Rep. 2018. PMID: 30120579 Review.
Cited by
-
Polyphenols and metabolism: from present knowledge to future challenges.J Physiol Biochem. 2024 Aug;80(3):603-625. doi: 10.1007/s13105-024-01046-7. Epub 2024 Oct 8. J Physiol Biochem. 2024. PMID: 39377969 Free PMC article. Review.
-
Nicotinamide Riboside, a Promising Vitamin B3 Derivative for Healthy Aging and Longevity: Current Research and Perspectives.Molecules. 2023 Aug 15;28(16):6078. doi: 10.3390/molecules28166078. Molecules. 2023. PMID: 37630330 Free PMC article. Review.
References
-
- WHO New WHO Report: Deaths from Noncommunicable Diseases on the Rise, with Developing World Hit Hardest. Cent. Eur. J. Public Health. 2011;19:114–120. - PubMed
-
- Duval C., Thissen U., Keshtkar S., Accart B., Stienstra R., Boekschoten M.V., Roskams T., Kersten S., Müller M. Adipose Tissue Dysfunction Signals Progression of Hepatic Steatosis towards Nonalcoholic Steatohepatitis in C57Bl/6 Mice. Diabetes. 2010;59:3181–3191. doi: 10.2337/db10-0224. - DOI - PMC - PubMed
-
- Mohamed S. Functional Foods against Metabolic Syndrome (Obesity, Diabetes, Hypertension and Dyslipidemia) and Cardiovasular Disease. Trends Food Sci. Technol. 2014;35:114–128. doi: 10.1016/j.tifs.2013.11.001. - DOI
-
- Petta S., Amato M.C., di Marco V., Cammà C., Pizzolanti G., Barcellona M.R., Cabibi D., Galluzzo A., Sinagra D., Giordano C., et al. Visceral Adiposity Index Is Associated with Significant Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. Aliment. Pharmacol. Ther. 2012;35:238–247. doi: 10.1111/j.1365-2036.2011.04929.x. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

