1,2,3-Triazole Derivatives as Novel Antifibrinolytic Drugs
- PMID: 36499270
- PMCID: PMC9736318
- DOI: 10.3390/ijms232314942
1,2,3-Triazole Derivatives as Novel Antifibrinolytic Drugs
Abstract
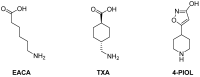
Fibrinolysis is a natural process that ensures blood fluidity through the removal of fibrin deposits. However, excessive fibrinolytic activity can lead to complications in different circumstances, such as general surgery or severe trauma. The current antifibrinolytic drugs in the market, aminocaproic acid (EACA) and tranexamic acid (TXA), require high doses repetitively to maintain their therapeutic effect. These high doses are related to a number of side effects such as headaches, nasal symptoms, or gastrointestinal discomfort and severely limit their use in patients with renal impairment. Therefore, the discovery of novel antifibrinolytics with a higher specificity and lower dosage could vastly improve the applicability of these drugs. Herein, we synthesized a total of ten compounds consisting of a combination of three key moieties: an oxadiazolone, a triazole, and a terminal amine. The IC50 of each compound was calculated in our clot lysis assays, and the best candidate (1) provided approximately a 2.5-fold improvement over the current gold standard, TXA. Molecular docking and molecular dynamics were used to perform a structure-activity relationship (SAR) analysis with the lysine binding site in the Kringle 1 domain of plasminogen. This analysis revealed that 1,2,3-triazole was crucial for the activity, enhancing the binding affinity through pi-pi stacking and polar interactions with Tyr72. The results presented in this work open the door to further investigate this new family as potential antifibrinolytic drugs.
Keywords: antifibrinolytic; fibrinolysis; oxadiazolone; plasmin; plasminogen; tPa; triazole.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








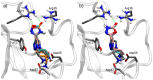




References
-
- Medved L., Nieuwenhuizen W. Molecular mechanisms of initiation of fibrinolysis by fibrin. Thromb. Haemost. 2003;89:409–419. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

