B4GALT1 as a New Biomarker of Idiopathic Pulmonary Fibrosis
- PMID: 36499368
- PMCID: PMC9738382
- DOI: 10.3390/ijms232315040
B4GALT1 as a New Biomarker of Idiopathic Pulmonary Fibrosis
Abstract
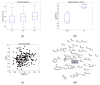
Idiopathic pulmonary fibrosis (IPF) is a disease characterized by progressive scarring of the lung that involves the pulmonary interstitium. The disease may rapidly progress, leading to respiratory failure, and the long-term survival is poor. There are no accurate biomarkers available so far. Our aim was to evaluate the expression of the B4GALT1 in patients with IPF. Analysis of B4GALT1 gene expression was performed in silico on two gene sets, retrieved from the Gene Expression Omnibus database. Expression of B4GALT1 was then evaluated, both at the mRNA and protein levels, on lung specimens obtained from lung biopsies of 4 IPF patients, on one IPF-derived human primary cell and on 11 cases of IPF associated with cancer. In silico re-analysis demonstrated that the B4GALT1 gene was overexpressed in patients and human cell cultures with IPF (p = 0.03). Network analysis demonstrated that B4GALT1 upregulation was correlated with genes belonging to the EMT pathway (p = 0.01). The overexpression of B4GALT1 was observed, both at mRNA and protein levels, in lung biopsies of our four IPF patients and in the IPF-derived human primary cell, in other fibrotic non-lung tissues, and in IPF associated with cancer. In conclusion, our results indicate that B4GALT1 is overexpressed in IPF and could represent a novel marker of this disease.
Keywords: B4GALT1; EMT; idiopathic fibrosis pulmonary; immunohistochemistry; lung cancer; lung fibrosis; mRNA.
Conflict of interest statement
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures



References
-
- Calabrese F., Lunardi F., Tauro V., Pezzuto F., Fortarezza F., Vedovelli L., Faccioli E., Balestro E., Schiavon M., Esposito G., et al. RNA Sequencing of Epithelial Cell/Fibroblastic Foci Sandwich in Idiopathic Pulmonary Fibrosis: New Insights on the Signaling Pathway. Int. J. Mol. Sci. 2022;23:3323. doi: 10.3390/ijms23063323. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

