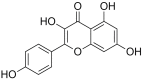
Kaempferol: Antimicrobial Properties, Sources, Clinical, and Traditional Applications
- PMID: 36499380
- PMCID: PMC9740324
- DOI: 10.3390/ijms232315054
Kaempferol: Antimicrobial Properties, Sources, Clinical, and Traditional Applications
Abstract
Flavonoids are a category of plant-derived compounds which exhibit a large number of health-related effects. One of the most well-known and studied flavonoids is kaempferol, which can be found in a wide variety of herbs and plant families. Apart from their anticarcinogenic and anti-inflammatory effects, kaempferol and its associated compounds also exhibit antibacterial, antifungal, and antiprotozoal activities. The development of drugs and treatment schemes based on these compounds is becoming increasingly important in the face of emerging resistance of numerous pathogens as well as complex molecular interactions between various drug therapies. In addition, many of the kaempferol-containing plants are used in traditional systems all over the world for centuries to treat numerous conditions. Due to its variety of sources and associated compounds, some molecular mechanisms of kaempferol antimicrobial activity are well known while others are still under analysis. This paper thoroughly documents the vegetal and food sources of kaempferol as well as the most recent and significant studies regarding its antimicrobial applications.
Keywords: antibacterial; antifungal; antiprotozoal; herbal medicine; kaempferol; molecular mechanisms.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources