Human β-Defensin 3 Inhibits Porphyromonas Gingivalis Lipopolysaccharide-Induced Oxidative and Inflammatory Responses of Microglia by Suppression of Cathepsins B and L
- PMID: 36499428
- PMCID: PMC9738813
- DOI: 10.3390/ijms232315099
Human β-Defensin 3 Inhibits Porphyromonas Gingivalis Lipopolysaccharide-Induced Oxidative and Inflammatory Responses of Microglia by Suppression of Cathepsins B and L
Abstract
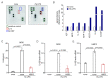
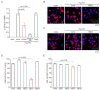
Recently, the effects of antibacterial peptides are suggested to have therapeutic potential in Alzheimer's disease. Furthermore, systemic treatment of Porphyromonas gingivalis (Pg) lipopolysaccharide (LPS) induced Alzheimer's disease-like neuropathological changes in middle-aged mice. Then, we examined whether human β-defensins (hBDs), antimicrobial peptides produced by the oral mucosa and salivary glands, can suppress Pg LPS-induced oxidative and inflammatory responses by microglia. hBD3 (1 μM) significantly suppressed Pg LPS-induced production of nitric oxide and interleukin-6 (IL-6) by MG6 cells, a mouse microglial cell line. hBD3 (1 μM) also significantly inhibited Pg LPS-induced expression of IL-6 by HMC3 cells, a human microglial cell line. In contrast, neither hBD1, hBD2 nor hBD4 failed to inhibit their productions. Furthermore, hBD3 suppressed Pg LPS-induced p65 nuclear translocation through the IκBα degradation. Pg LPS-induced expression of IL-6 was significantly suppressed by E64d, a cysteine protease inhibitor, and CA-074Me, a known specific inhibitor for cathepsin B, but not by pepstatin A, an aspartic protease inhibitor. Interestingly, hBD3 significantly inhibited enzymatic activities of recombinant human cathepsins B and L, lysosomal cysteine proteases, and their intracellular activities in MG6 cells. Therefore, hBD3 suppressed oxidative and inflammatory responses of microglia through the inhibition of cathepsins B and L, which enzymatic activities are necessary for the NF-κB activation.
Keywords: NF-κB p65; Porphyromonas gingivalis; antibacterial peptide; cathepsin B; cathepsin L; human β-defensins; interleukin-6; lipopolysaccharide; microglia; nitric oxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Human β-Defensin 3 Inhibition of P. gingivalis LPS-Induced IL-1β Production by BV-2 Microglia through Suppression of Cathepsins B and L.Cells. 2024 Feb 4;13(3):283. doi: 10.3390/cells13030283. Cells. 2024. PMID: 38334675 Free PMC article.
-
Noradrenaline Synergistically Enhances Porphyromonas gingivalis LPS and OMV-Induced Interleukin-1β Production in BV-2 Microglia Through Differential Mechanisms.Int J Mol Sci. 2025 Mar 15;26(6):2660. doi: 10.3390/ijms26062660. Int J Mol Sci. 2025. PMID: 40141302 Free PMC article.
-
Cathepsin B plays a critical role in inducing Alzheimer's disease-like phenotypes following chronic systemic exposure to lipopolysaccharide from Porphyromonas gingivalis in mice.Brain Behav Immun. 2017 Oct;65:350-361. doi: 10.1016/j.bbi.2017.06.002. Epub 2017 Jun 10. Brain Behav Immun. 2017. PMID: 28610747
-
Microglial Cathepsin B and Porphyromonas gingivalis Gingipains as Potential Therapeutic Targets for Sporadic Alzheimer's Disease.CNS Neurol Disord Drug Targets. 2020;19(7):495-502. doi: 10.2174/1871527319666200708125130. CNS Neurol Disord Drug Targets. 2020. PMID: 32640970 Review.
-
Cysteine Cathepsins in the secretory vesicle produce active peptides: Cathepsin L generates peptide neurotransmitters and cathepsin B produces beta-amyloid of Alzheimer's disease.Biochim Biophys Acta. 2012 Jan;1824(1):89-104. doi: 10.1016/j.bbapap.2011.08.015. Epub 2011 Sep 8. Biochim Biophys Acta. 2012. PMID: 21925292 Free PMC article. Review.
Cited by
-
Human β-Defensin 3 Inhibition of P. gingivalis LPS-Induced IL-1β Production by BV-2 Microglia through Suppression of Cathepsins B and L.Cells. 2024 Feb 4;13(3):283. doi: 10.3390/cells13030283. Cells. 2024. PMID: 38334675 Free PMC article.
-
Alzheimer's Disease and Porphyromonas gingivalis: Exploring the Links.Life (Basel). 2025 Jan 14;15(1):96. doi: 10.3390/life15010096. Life (Basel). 2025. PMID: 39860036 Free PMC article. Review.
-
Noradrenaline Synergistically Enhances Porphyromonas gingivalis LPS and OMV-Induced Interleukin-1β Production in BV-2 Microglia Through Differential Mechanisms.Int J Mol Sci. 2025 Mar 15;26(6):2660. doi: 10.3390/ijms26062660. Int J Mol Sci. 2025. PMID: 40141302 Free PMC article.
-
Proteomic analysis of P. gingivalis-Lipopolysaccharide induced neuroinflammation in SH-SY5Y and HMC3 cells.Geroscience. 2024 Oct;46(5):4315-4332. doi: 10.1007/s11357-024-01117-z. Epub 2024 Mar 20. Geroscience. 2024. PMID: 38507186 Free PMC article.
-
Triptolide improves Alzheimer's disease by regulating the NF‑κB signaling pathway through the lncRNA NEAT1/microRNA 361‑3p/TRAF2 axis.Exp Ther Med. 2023 Aug 1;26(3):440. doi: 10.3892/etm.2023.12139. eCollection 2023 Sep. Exp Ther Med. 2023. PMID: 37614428 Free PMC article.
References
-
- Liu Y., Wu Z., Nakanishi Y., Ni J., Hayashi Y., Takayama F., Zhou Y., Kadowaki T., Nakanishi H. Infection of microglia with Porphyromonas gingivalis promotes cell migration and an inflammatory response through the gingipain-mediated activation of protease-activated receptor-2 in mice. Sci. Rep. 2017;7:11759. doi: 10.1038/s41598-017-12173-1. - DOI - PMC - PubMed
-
- Nonaka S., Kadowaki T., Nakanishi H. Secreted gingipains from Porphyromonas gingivalis increase permeability in human cerebral microvascular endothelial cells through intracellular degradation of tight junction proteins. Neurochem. Int. 2022;154:105282. doi: 10.1016/j.neuint.2022.105282. - DOI - PubMed
-
- Dominy S.S., Lynch C., Ermini F., Benedyk M., Marczyk A., Konradi A., Nguyen M., Haditsch U., Raha D., Griffin C., et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019;5:eaau3333. doi: 10.1126/sciadv.aau3333. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

