Functional Effects of Epilepsy Associated KCNT1 Mutations Suggest Pathogenesis via Aberrant Inhibitory Neuronal Activity
- PMID: 36499459
- PMCID: PMC9740882
- DOI: 10.3390/ijms232315133
Functional Effects of Epilepsy Associated KCNT1 Mutations Suggest Pathogenesis via Aberrant Inhibitory Neuronal Activity
Abstract
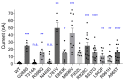
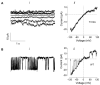
KCNT1 (K+ channel subfamily T member 1) is a sodium-activated potassium channel highly expressed in the nervous system which regulates neuronal excitability by contributing to the resting membrane potential and hyperpolarisation following a train of action potentials. Gain of function mutations in the KCNT1 gene are the cause of neurological disorders associated with different forms of epilepsy. To gain insights into the underlying pathobiology we investigated the functional effects of 9 recently published KCNT1 mutations, 4 previously studied KCNT1 mutations, and one previously unpublished KCNT1 variant of unknown significance. We analysed the properties of KCNT1 potassium currents and attempted to find a correlation between the changes in KCNT1 characteristics due to the mutations and severity of the neurological disorder they cause. KCNT1 mutations identified in patients with epilepsy were introduced into the full length human KCNT1 cDNA using quick-change site-directed mutagenesis protocol. Electrophysiological properties of different KCNT1 constructs were investigated using a heterologous expression system (HEK293T cells) and patch clamping. All mutations studied, except T314A, increased the amplitude of KCNT1 currents, and some mutations shifted the voltage dependence of KCNT1 open probability, increasing the proportion of channels open at the resting membrane potential. The T314A mutation did not affect KCNT1 current amplitude but abolished its voltage dependence. We observed a positive correlation between the severity of the neurological disorder and the KCNT1 channel open probability at resting membrane potential. This suggests that gain of function KCNT1 mutations cause epilepsy by increasing resting potassium conductance and suppressing the activity of inhibitory neurons. A reduction in action potential firing in inhibitory neurons due to excessively high resting potassium conductance leads to disinhibition of neural circuits, hyperexcitability and seizures.
Keywords: K+ channels; channelopathies; epilepsy; gain-of-function mutations; patch clamping.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures






References
-
- Heron S.E., Smith K.R., Bahlo M., Nobili L., Kahana E., Licchetta L., Oliver K.L., Mazarib A., Afawi Z., Korczyn A., et al. Missense mutations in the sodium-gated potassium channel gene KCNT1 cause severe autosomal dominant nocturnal frontal lobe epilepsy. Nat. Genet. 2012;44:1188–1190. doi: 10.1038/ng.2440. - DOI - PubMed
-
- Barcia G., Fleming M.R., Deligniere A., Gazula V.R., Brown M.R., Langouet M., Chen H.J., Kronengold J., Abhyankar A., Cilio R., et al. De novo gain-of-function KCNT1 channel mutations cause malignant migrating partial seizures of infancy. Nat. Genet. 2012;44:1255–1259. doi: 10.1038/ng.2441. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

