Resveratrol-like Compounds as SIRT1 Activators
- PMID: 36499460
- PMCID: PMC9738298
- DOI: 10.3390/ijms232315105
Resveratrol-like Compounds as SIRT1 Activators
Abstract
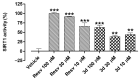
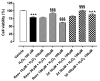
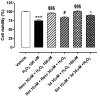
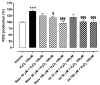
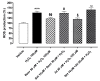
The sirtuin 1 (SIRT1) activator resveratrol has emerged as a promising candidate for the prevention of vascular oxidative stress, which is a trigger for endothelial dysfunction. However, its clinical use is limited by low oral bioavailability. In this work, we have applied a previously developed computational protocol to identify the most promising derivatives from our in-house chemical library of resveratrol derivatives. The most promising compounds in terms of SIRT1 activation and oral bioavailability, predicted in silico, were evaluated for their ability to activate the isolated SIRT1 enzyme. Then, we assessed the antioxidant effects of the most effective derivative, compound 3d, in human umbilical vein endothelial cells (HUVECs) injured with H2O2 100 µM. The SIRT1 activator 3d significantly preserved cell viability and prevented an intracellular reactive oxygen species increase in HUVECs exposed to the oxidative stimulus. Such effects were partially reduced in the presence of a sirtuin inhibitor, sirtinol, confirming the potential role of sirtuins in the activity of resveratrol and its derivatives. Although 3d appeared less effective than resveratrol in activating the isolated enzyme, the effects exhibited by both compounds in HUVECs were almost superimposable, suggesting a higher ability of 3d to cross cell membranes and activate the intracellular target SIRT1.
Keywords: SIRT1 activators; computer aided drug discovery; endothelial dysfunction; nature-inspired compounds; oxidative stress; resveratrol; resveratrol-like compounds; sirtuin 1; structure-activity relationship (SAR); vascular endothelium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Resveratrol protects human endothelium from H(2)O(2)-induced oxidative stress and senescence via SirT1 activation.J Atheroscler Thromb. 2010 Sep 30;17(9):970-9. doi: 10.5551/jat.4333. Epub 2010 Jul 13. J Atheroscler Thromb. 2010. PMID: 20644332
-
Screening Analysis of Sirtuins Family Expression on Anti-Inflammation of Resveratrol in Endothelial Cells.Med Sci Monit. 2019 Jun 3;25:4137-4148. doi: 10.12659/MSM.913240. Med Sci Monit. 2019. PMID: 31158122 Free PMC article.
-
Resveratrol prevents oxidative stress-induced senescence and proliferative dysfunction by activating the AMPK-FOXO3 cascade in cultured primary human keratinocytes.PLoS One. 2015 Feb 3;10(2):e0115341. doi: 10.1371/journal.pone.0115341. eCollection 2015. PLoS One. 2015. PMID: 25647160 Free PMC article.
-
Sirtuins, resveratrol and the intertwining cellular pathways connecting them.Ageing Res Rev. 2023 Jul;88:101936. doi: 10.1016/j.arr.2023.101936. Epub 2023 Apr 26. Ageing Res Rev. 2023. PMID: 37116286 Review.
-
Sirtuin activators.Expert Opin Ther Pat. 2009 Apr;19(4):403-14. doi: 10.1517/13543770902762893. Expert Opin Ther Pat. 2009. PMID: 19441923 Review.
Cited by
-
Sphingolipid Levels and Signaling via Resveratrol and Antioxidant Actions in Cardiometabolic Risk and Disease.Antioxidants (Basel). 2023 May 16;12(5):1102. doi: 10.3390/antiox12051102. Antioxidants (Basel). 2023. PMID: 37237968 Free PMC article. Review.
-
Recent Advances in Resveratrol Derivatives: Structural Modifications and Biological Activities.Molecules. 2025 Feb 19;30(4):958. doi: 10.3390/molecules30040958. Molecules. 2025. PMID: 40005268 Free PMC article. Review.
-
Immunomodulatory and Antioxidant Drugs in Glaucoma Treatment.Pharmaceuticals (Basel). 2023 Aug 22;16(9):1193. doi: 10.3390/ph16091193. Pharmaceuticals (Basel). 2023. PMID: 37765001 Free PMC article. Review.
-
Recent Advances in the Discovery of SIRT1/2 Inhibitors via Computational Methods: A Perspective.Pharmaceuticals (Basel). 2024 May 8;17(5):601. doi: 10.3390/ph17050601. Pharmaceuticals (Basel). 2024. PMID: 38794171 Free PMC article. Review.
-
Resveratrol and Vitamin D: Eclectic Molecules Promoting Mitochondrial Health in Sarcopenia.Int J Mol Sci. 2024 Jul 9;25(14):7503. doi: 10.3390/ijms25147503. Int J Mol Sci. 2024. PMID: 39062745 Free PMC article. Review.
References
-
- Steven S., Frenis K., Oelze M., Kalinovic S., Kuntic M., Bayo Jimenez M.T., Vujacic-Mirski K., Helmstädter J., Kröller-Schön S., Münzel T., et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxidative Med. Cell. Longev. 2019;2019:e7092151. doi: 10.1155/2019/7092151. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

