Pregnancy Increases CYP3A Enzymes Activity as Measured by the 4β-Hydroxycholesterol/Cholesterol Ratio
- PMID: 36499500
- PMCID: PMC9739497
- DOI: 10.3390/ijms232315168
Pregnancy Increases CYP3A Enzymes Activity as Measured by the 4β-Hydroxycholesterol/Cholesterol Ratio
Abstract
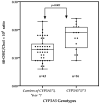
Changes in cortisol and other hormones during pregnancy may alter CYP3A enzymes activity, but data from sub-Saharan Africa are sparse. We investigated the effect of pregnancy and CYP3A5 genotypes on CYP3A enzymes activity using the plasma 4β-hydroxycholesterol (4β-OHC)/cholesterol (Chol) ratio, a known endogenous biomarker. Tanzanian pregnant women (n = 110) and non-pregnant women (n = 59) controls were enrolled. Plasma 4β-OHC and Chol were determined in the second and third trimesters for pregnant women and once for non-pregnant women using gas chromatography−mass spectrometry. Genotyping for CYP3A5 (*3, *6, *7) was performed. Wilcoxon Signed-Rank Test and Mann−Whitney U test were used to compare the median 4β-OHC/Chol ratio between trimesters in pregnant women and between pregnant and non-pregnant women. Repeated-measure ANOVA was used to evaluate the effect of the CYP3A5 genotypes on the 4β-OHC/Chol ratio in pregnant women. No significant effect of the pregnancy status or the CYP3A5 genotype on the cholesterol level was observed. The plasma 4β-OHC/Chol ratio significantly increased by 7.3% from the second trimester to the third trimester (p = 0.02). Pregnant women had a significantly higher mean 4β-OHC/Chol ratio than non-pregnant women, (p < 0.001). In non-pregnant women, the mean 4β-OHC/Chol ratio was significantly lower in carriers of defective CYP3A5 alleles (*3, *6 or *7) as compared to women with the CYP3A5*1/*1 genotypes (p = 0.002). Pregnancy increases CYP3A enzymes activity in a gestational-stage manner. The CYP3A5 genotype predicts CYP3A enzymes activity in the black Tanzanian population, but not during pregnancy-mediated CYP3A enzyme induction.
Keywords: 4β-hydroxycholesterol/cholesterol; CYP3A; pregnancy; trimester.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


Similar articles
-
Sex and CYP3A5 genotype influence total CYP3A activity: high CYP3A activity and a unique distribution of CYP3A5 variant alleles in Ethiopians.Pharmacogenomics J. 2011 Apr;11(2):130-7. doi: 10.1038/tpj.2010.16. Epub 2010 Mar 16. Pharmacogenomics J. 2011. PMID: 20231858
-
Evaluation of effects of indoxyl sulfate and parathyroid hormone on CYP3A activity considering the influence of CYP3A5 gene polymorphisms.Br J Clin Pharmacol. 2023 Dec;89(12):3648-3658. doi: 10.1111/bcp.15866. Epub 2023 Aug 22. Br J Clin Pharmacol. 2023. PMID: 37522799
-
Evaluation of the usefulness of plasma 4β-hydroxycholesterol concentration normalized by 4α-hydroxycholesterol for accurate CYP3A phenotyping.Clin Transl Sci. 2024 Mar;17(3):e13768. doi: 10.1111/cts.13768. Clin Transl Sci. 2024. PMID: 38465776 Free PMC article.
-
4β-Hydroxycholesterol, an endogenous marker of CYP3A4/5 activity in humans.Br J Clin Pharmacol. 2011 Feb;71(2):183-9. doi: 10.1111/j.1365-2125.2010.03773.x. Br J Clin Pharmacol. 2011. PMID: 21219398 Free PMC article. Review.
-
4β-Hydroxycholesterol as an Endogenous Biomarker for CYP3A Activity: Literature Review and Critical Evaluation.J Clin Pharmacol. 2019 May;59(5):611-624. doi: 10.1002/jcph.1391. Epub 2019 Feb 12. J Clin Pharmacol. 2019. PMID: 30748026 Review.
Cited by
-
CYP3A4 and CYP3A5: the crucial roles in clinical drug metabolism and the significant implications of genetic polymorphisms.PeerJ. 2024 Dec 5;12:e18636. doi: 10.7717/peerj.18636. eCollection 2024. PeerJ. 2024. PMID: 39650550 Free PMC article. Review.
-
Innovations, Opportunities, and Challenges for Predicting Alteration in Drug-Metabolizing Enzyme and Transporter Activity in Specific Populations.Drug Metab Dispos. 2023 Dec;51(12):1547-1550. doi: 10.1124/dmd.123.001453. Epub 2023 Sep 29. Drug Metab Dispos. 2023. PMID: 37775331 Free PMC article.
-
Pregnancy related hormones increase CYP3A mediated buprenorphine metabolism in human hepatocytes: a comparison to CYP3A substrates nifedipine and midazolam.Front Pharmacol. 2023 Jul 5;14:1218703. doi: 10.3389/fphar.2023.1218703. eCollection 2023. Front Pharmacol. 2023. PMID: 37475714 Free PMC article.
-
Impact of sex and pregnancy on hepatic CYP3A4 expression and activity in a humanized mouse model.Drug Metab Dispos. 2025 Feb;53(2):100025. doi: 10.1016/j.dmd.2024.100025. Epub 2024 Nov 29. Drug Metab Dispos. 2025. PMID: 40023573
-
Pharmacokinetics of piperaquine and its association with intermittent malaria preventive therapy outcomes during pregnancy.BMC Pharmacol Toxicol. 2024 Jul 8;25(1):38. doi: 10.1186/s40360-024-00762-6. BMC Pharmacol Toxicol. 2024. PMID: 38978151 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

