Endoplasmic Reticulum Stress Signaling and Neuronal Cell Death
- PMID: 36499512
- PMCID: PMC9740965
- DOI: 10.3390/ijms232315186
Endoplasmic Reticulum Stress Signaling and Neuronal Cell Death
Abstract
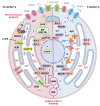
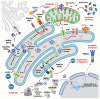
Besides protein processing, the endoplasmic reticulum (ER) has several other functions such as lipid synthesis, the transfer of molecules to other cellular compartments, and the regulation of Ca2+ homeostasis. Before leaving the organelle, proteins must be folded and post-translationally modified. Protein folding and revision require molecular chaperones and a favorable ER environment. When in stressful situations, ER luminal conditions or chaperone capacity are altered, and the cell activates signaling cascades to restore a favorable folding environment triggering the so-called unfolded protein response (UPR) that can lead to autophagy to preserve cell integrity. However, when the UPR is disrupted or insufficient, cell death occurs. This review examines the links between UPR signaling, cell-protective responses, and death following ER stress with a particular focus on those mechanisms that operate in neurons.
Keywords: apoptosis; autophagy; cerebellar granule cells; endoplasmic reticulum; endoplasmic reticulum stress; unfolded protein response.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

